Nanovesicles from adipose-derived mesenchymal stem cells inhibit T lymphocyte trafficking and ameliorate chronic experimental autoimmune encephalomyelitis
- PMID: 29748664
- PMCID: PMC5945853
- DOI: 10.1038/s41598-018-25676-2
Nanovesicles from adipose-derived mesenchymal stem cells inhibit T lymphocyte trafficking and ameliorate chronic experimental autoimmune encephalomyelitis
Abstract
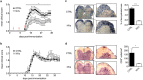
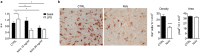
Cell based-therapies represent promising strategies for the treatment of neurological diseases. We have previously shown that adipose stem cells (ASC) ameliorate chronic experimental autoimmune encephalomyelitis (EAE). Recent evidence indicates that most ASC paracrine effects are mediated by extracellular vesicles, i.e. micro- and nanovesicles (MVs and NVs). We show that preventive intravenous administration of NVs isolated from ASC (ASC-NVs) before disease onset significantly reduces the severity of EAE and decreases spinal cord inflammation and demyelination, whereas therapeutic treatment with ASC-NVs does not ameliorate established EAE. This treatment marginally inhibits antigen-specific T cell activation, while reducing microglial activation and demyelination in the spinal cord. Importantly, ASC-NVs inhibited integrin-dependent adhesion of encephalitogenic T cells in vitro, with no effect on adhesion molecule expression. In addition, intravital microscopy showed that encephalitogenic T cells treated with ASC NVs display a significantly reduced rolling and firm adhesion in inflamed spinal cord vessels compared to untreated cells. Our results show that ASC-NVs ameliorate EAE pathogenesis mainly by inhibiting T cell extravasation in the inflamed CNS, suggesting that NVs may represent a novel therapeutic approach in neuro-inflammatory diseases, enabling the safe administration of ASC effector factors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Raine, C. S. The neuropathology of multiple sclerosis. In: Raine, C. S., McFarland, H. & Tourtellotte, W. eds Multiple sclerosis 149–172 (London, UK: Chapman & Hall Med, 1997).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

