Effects of Delta-tocotrienol Supplementation on Liver Enzymes, Inflammation, Oxidative stress and Hepatic Steatosis in Patients with Nonalcoholic Fatty Liver Disease
- PMID: 29749323
- PMCID: PMC6284694
- DOI: 10.5152/tjg.2018.17297
Effects of Delta-tocotrienol Supplementation on Liver Enzymes, Inflammation, Oxidative stress and Hepatic Steatosis in Patients with Nonalcoholic Fatty Liver Disease
Abstract
Background/aims: Non-alcoholic fatty liver disease (NAFLD) is a growing public health problem worldwide and is associated with increased morbidity and mortality. Currently, there is no definitive treatment for this disease. δ-Tocotrienol has potent anti-inflammatory and antioxidant properties and may reduce liver injury in NAFLD. The present study aims to evaluate the efficacy and safety of δ-tocotrienol in the treatment of NAFLD.
Materials and methods: The present study was a randomized, double-blind, placebo-controlled pilot study conducted in patients aged > 20 years, belonging to both sexes, having ultrasound-proven fatty liver disease, having a fatty liver index (FLI) of ≥ 60, and persistent elevation of alanine transaminase. A total of 71 patients were assigned to receive either oral δ-tocotrienol (n=35, 300 mg twice daily) or placebo (n=36) for 12 weeks. At the baseline and at the end of the study, clinical and biochemical parameters, including lipid profile, liver function tests, high-sensitivity C-reactive protein (hs-CRP), and malondialdehyde (MDA) were measured. Body mass index and FLI were calculated, and ultrasound grading of hepatic steatosis was performed.
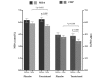
Results: Out of 71 enrolled patients, 64 patients, 31 in the δ-tocotrienol group and 33 in the placebo group, completed the study. After 12 weeks of supplementation, δ-tocotrienol showed greater efficacy than placebo by decreasing serum aminotransferases, hs-CRP, MDA, and FLI score (p<0.001). However, it did not improve hepatic steatosis on ultrasound examination. No adverse effects were reported.
Conclusion: δ-Tocotrienol was safe, and it effectively improved aminotransferase levels and inflammatory and oxidative stress markers in patients with NAFLD. Large-scale randomized clinical trials are warranted to further support these findings.
Conflict of interest statement
Figures

References
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. https://doi.org/10.1053/j.gastro.2012.04.001 - DOI - PubMed
-
- Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37:97–103. https://doi.org/10.1111/liv.13302 - DOI - PMC - PubMed
-
- Koplay M, Sivri M, Erdogan H, Nayman A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:769–76. https://doi.org/10.4254/wjh.v7.i5.769 - DOI - PMC - PubMed
-
- AlShaalan R, Aljiffry M, Al-Busafi S, Metrakos P, Hassanain M. Nonalcoholic Fatty Liver Disease: Noninvasive Methods of Diagnosing Hepatic Steatosis. Saudi J Gastroenterol. 2015;21:64–70. https://doi.org/10.4103/1319-3767.153812 - DOI - PMC - PubMed
-
- Zelber-Sagi S, Webb M, Assy N, et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J Gastroenterol. 2013;19:57–64. https://doi.org/10.3748/wjg.v19.i1.57 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
