Automated FES for Upper Limb Rehabilitation Following Stroke and Spinal Cord Injury
- PMID: 29752242
- PMCID: PMC6051484
- DOI: 10.1109/TNSRE.2018.2816238
Automated FES for Upper Limb Rehabilitation Following Stroke and Spinal Cord Injury
Abstract
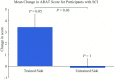
Neurorehabilitation aims to induce beneficial neural plasticity in order to restore function following injury to the nervous system. There is an increasing evidence that appropriately timed functional electrical stimulation (FES) can promote associative plasticity, but the dosage is critical for lasting functional benefits. Here, we present a novel approach to closed-loop control of muscle stimulation for the rehabilitation of reach-to-grasp movements following stroke and spinal cord injury (SCI). We developed a simple, low-cost device to deliver assistive stimulation contingent on users' self-initiated movements. The device allows repeated practice with minimal input by a therapist, and is potentially suitable for home use. Pilot data demonstrate usability by people with upper limb weakness following SCI and stroke, and participant feedback was positive. Moreover, repeated training with the device over 1-2 weeks led to functional benefits on a general object manipulation assessment. Thus, automated FES delivered by this novel device may provide a promising and readily translatable therapy for upper limb rehabilitation for people with stroke and SCI.
Figures






Similar articles
-
Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients.ScientificWorldJournal. 2007 Dec 20;7:2031-45. doi: 10.1100/tsw.2007.299. ScientificWorldJournal. 2007. PMID: 18167618 Free PMC article.
-
End-user and clinician perspectives on the viability of wearable functional electrical stimulation garments after stroke and spinal cord injury.Disabil Rehabil Assist Technol. 2021 Apr;16(3):241-250. doi: 10.1080/17483107.2019.1668974. Epub 2019 Oct 8. Disabil Rehabil Assist Technol. 2021. PMID: 31592679
-
Synergy-Based FES for Post-Stroke Rehabilitation of Upper-Limb Motor Functions.IEEE Trans Neural Syst Rehabil Eng. 2019 Feb;27(2):256-264. doi: 10.1109/TNSRE.2019.2891004. IEEE Trans Neural Syst Rehabil Eng. 2019. PMID: 30763238
-
A review of methods for achieving upper limb movement following spinal cord injury through hybrid muscle stimulation and robotic assistance.Exp Neurol. 2020 Jun;328:113274. doi: 10.1016/j.expneurol.2020.113274. Epub 2020 Mar 5. Exp Neurol. 2020. PMID: 32145251 Review.
-
Development of Functional Electrical Stimulation Rowing: The Rowstim Series.Artif Organs. 2017 Nov;41(11):E203-E212. doi: 10.1111/aor.13053. Artif Organs. 2017. PMID: 29148129 Review.
Cited by
-
Recognition of Human Lower Limb Motion and Muscle Fatigue Status Using a Wearable FES-sEMG System.Sensors (Basel). 2024 Apr 8;24(7):2377. doi: 10.3390/s24072377. Sensors (Basel). 2024. PMID: 38610589 Free PMC article.
-
Decoding hand and wrist movement intention from chronic stroke survivors with hemiparesis using a user-friendly, wearable EMG-based neural interface.J Neuroeng Rehabil. 2024 Jan 13;21(1):7. doi: 10.1186/s12984-023-01301-w. J Neuroeng Rehabil. 2024. PMID: 38218901 Free PMC article.
-
Reliability of Wearable-Sensor-Derived Measures of Physical Activity in Wheelchair-Dependent Spinal Cord Injured Patients.Front Neurol. 2018 Dec 10;9:1039. doi: 10.3389/fneur.2018.01039. eCollection 2018. Front Neurol. 2018. PMID: 30619026 Free PMC article.
-
A Co-driven Functional Electrical Stimulation Control Strategy by Dynamic Surface Electromyography and Joint Angle.Front Neurosci. 2022 Jul 8;16:909602. doi: 10.3389/fnins.2022.909602. eCollection 2022. Front Neurosci. 2022. PMID: 35898409 Free PMC article.
-
Evaluation of a conducting elastomeric composite material for intramuscular electrode application.Acta Biomater. 2020 Feb;103:81-91. doi: 10.1016/j.actbio.2019.12.021. Epub 2019 Dec 18. Acta Biomater. 2020. PMID: 31863910 Free PMC article.
References
-
- National Spinal Cord Injury Statistical Center, “Spinal cord injury (SCI) 2016 facts and figures at a glance,” J. Spinal Cord Med., vol. 39, no. 4, pp. 493–494, Jul. 2016. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/27471859 - PMC - PubMed
-
- Anderson K. D., “Targeting recovery: Priorities of the spinal cord-injured population,” J. Neurotrauma, vol. 21, no. 10, pp. 1371–1383, Oct. 2004. - PubMed
-
- Welmer A. K., Holmqvist L. W., and Sommerfeld D. K., “Limited fine hand use after stroke and its association with other disabilities,” J. Rehabil. Med., vol. 40, no. 8, pp. 603–608, Aug. 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

