Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5
- PMID: 29752311
- PMCID: PMC6008229
- DOI: 10.4049/jimmunol.1701069
Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5
Abstract
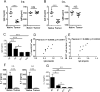
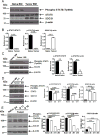
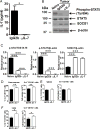
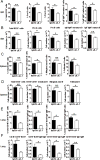
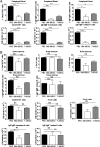
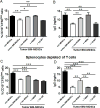
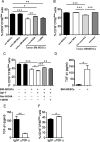
Myeloid-derived suppressor cells (MDSCs) are known suppressors of antitumor immunity, affecting amino acid metabolism and T cell function in the tumor microenvironment. However, it is unknown whether MDSCs regulate B cell responses during tumor progression. Using a syngeneic mouse model of lung cancer, we show reduction in percentages and absolute numbers of B cell subsets including pro-, pre-, and mature B cells in the bone marrow (BM) of tumor-bearing mice. The kinetics of this impaired B cell response correlated with the progressive infiltration of MDSCs. We identified that IL-7 and downstream STAT5 signaling that play a critical role in B cell development and differentiation were also impaired during tumor progression. Global impairment of B cell function was indicated by reduced serum IgG levels. Importantly, we show that anti-Gr-1 Ab-mediated depletion of MDSCs not only rescued serum IgG and IL-7 levels but also reduced TGF-β1, a known regulator of stromal IL-7, suggesting MDSC-mediated regulation of B cell responses. Furthermore, blockade of IL-7 resulted in reduced phosphorylation of downstream STAT5 and B cell differentiation in tumor-bearing mice and administration of TGF-β-blocking Ab rescued these IL-7-dependent B cell responses. Adoptive transfer of BM-derived MDSCs from tumor-bearing mice into congenic recipients resulted in significant reductions of B cell subsets in the BM and in circulation. MDSCs also suppressed B cell proliferation in vitro in an arginase-dependent manner that required cell-to-cell contact. Our results indicate that tumor-infiltrating MDSCs may suppress humoral immune responses and promote tumor escape from immune surveillance.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures





Comment in
-
Interplay between immune cells in lung cancer: beyond T lymphocytes.Transl Lung Cancer Res. 2018 Dec;7(Suppl 4):S336-S340. doi: 10.21037/tlcr.2018.11.05. Transl Lung Cancer Res. 2018. PMID: 30705849 Free PMC article. No abstract available.
-
Myeloid-derived suppressor cell-dependent inhibition of B cell responses in non-small cell lung cancer.Transl Lung Cancer Res. 2019 Dec;8(Suppl 4):S331-S333. doi: 10.21037/tlcr.2019.04.10. Transl Lung Cancer Res. 2019. PMID: 32038908 Free PMC article. No abstract available.
References
-
- Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91:493–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

