Prolonged duration of response in lenvatinib responders with thyroid cancer
- PMID: 29752332
- PMCID: PMC5958278
- DOI: 10.1530/ERC-18-0049
Prolonged duration of response in lenvatinib responders with thyroid cancer
Abstract
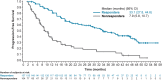
We present an updated analysis of lenvatinib in radioiodine-refractory differentiated thyroid cancer (RR-DTC) with new duration of response (DOR) data unavailable for the primary analysis. In this randomized, double-blind, multicenter, placebo-controlled phase 3 study, patients ≥18 years old with measurable, pathologically confirmed RR-DTC with independent radiologic confirmation of disease progression within the previous 13 months were randomized 2:1 to oral lenvatinib 24 mg/day or placebo. The main outcome measures for this analysis are DOR and progression-free survival (PFS). The median DOR for all lenvatinib responders (patients with complete or partial responses; objective response rate: 60.2%; 95% confidence interval (CI) 54.2-66.1) was 30.0 months (95% CI 18.4-36.7) and was generally similar across subgroups. DOR was shorter in patients with greater disease burden and with brain and liver metastases. Updated median PFS was longer in the overall lenvatinib group vs placebo (19.4 vs 3.7 months; hazard ratio (HR) 0.24; 99% CI 0.17-0.35; nominal P < 0.0001). In lenvatinib responders, median PFS was 33.1 months (95% CI 27.8-44.6) vs 7.9 months (95% CI 5.8-10.7) in non-responders. The median DOR of 30.0 months seen with patients who achieved complete or partial responses with lenvatinib (60.2%) demonstrates that lenvatinib responders can have prolonged, durable and clinically meaningful responses. Prolonged PFS (33.1 months) was also observed in these lenvatinib responders.
Keywords: SELECT; duration of response; lenvatinib; radioiodine-refractory differentiated thyroid carcinoma.
© 2018 The authors.
Figures
References
-
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, et al 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 319–328. (10.1016/S0140-6736(14)60421-9) - DOI - PMC - PubMed
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19 1167–1214. (10.1089/thy.2009.0110) - DOI - PubMed
-
- Dieci MV, Arnedos M, Andre F, Soria JC. 2013. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discovery 3 264–279. (10.1158/2159-8290.CD-12-0362) - DOI - PubMed
-
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, et al 2006. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. Journal of Clinical Endocrinology and Metabolism 91 2892–2899. (10.1210/jc.2005-2838) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical