Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation
- PMID: 29752413
- PMCID: PMC6050928
- DOI: 10.1681/ASN.2018010009
Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation
Abstract
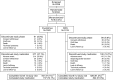
Background Everolimus permits reduced calcineurin inhibitor (CNI) exposure, but the efficacy and safety outcomes of this treatment after kidney transplant require confirmation.Methods In a multicenter noninferiority trial, we randomized 2037 de novo kidney transplant recipients to receive, in combination with induction therapy and corticosteroids, everolimus with reduced-exposure CNI (everolimus arm) or mycophenolic acid (MPA) with standard-exposure CNI (MPA arm). The primary end point was treated biopsy-proven acute rejection or eGFR<50 ml/min per 1.73 m2 at post-transplant month 12 using a 10% noninferiority margin.Results In the intent-to-treat population (everolimus n=1022, MPA n=1015), the primary end point incidence was 48.2% (493) with everolimus and 45.1% (457) with MPA (difference 3.2%; 95% confidence interval, -1.3% to 7.6%). Similar between-treatment differences in incidence were observed in the subgroups of patients who received tacrolimus or cyclosporine. Treated biopsy-proven acute rejection, graft loss, or death at post-transplant month 12 occurred in 14.9% and 12.5% of patients treated with everolimus and MPA, respectively (difference 2.3%; 95% confidence interval, -1.7% to 6.4%). De novo donor-specific antibody incidence at 12 months and antibody-mediated rejection rate did not differ between arms. Cytomegalovirus (3.6% versus 13.3%) and BK virus infections (4.3% versus 8.0%) were less frequent in the everolimus arm than in the MPA arm. Overall, 23.0% and 11.9% of patients treated with everolimus and MPA, respectively, discontinued the study drug because of adverse events.Conclusions In kidney transplant recipients at mild-to-moderate immunologic risk, everolimus was noninferior to MPA for a binary composite end point assessing immunosuppressive efficacy and preservation of graft function.
Trial registration: ClinicalTrials.gov NCT01950819.
Keywords: calcineurin inhibitor; efficacy graft; everolimus; function; kidney transplantation; randomized.
Copyright © 2018 by the American Society of Nephrology.
Figures


Comment in
-
Transformation in Immunosuppression: Are We Ready for it?J Am Soc Nephrol. 2018 Jul;29(7):1791-1792. doi: 10.1681/ASN.2018050491. Epub 2018 Jun 8. J Am Soc Nephrol. 2018. PMID: 29884733 Free PMC article. No abstract available.
References
-
- Gondos A, Döhler B, Brenner H, Opelz G: Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 95: 267–274, 2013 - PubMed
-
- Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al.: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 - PubMed
-
- Stegall MD, Cornell LD, Park WD, Smith BH, Cosio FG: Renal allograft histology at 10 years after transplantation in the tacrolimus era: Evidence of pervasive chronic injury. Am J Transplant 18: 180–188, 2018 - PubMed
-
- Einecke G, Reeve J, Halloran PF: Hyalinosis lesions in renal transplant biopsies: Time-dependent complexity of interpretation. Am J Transplant 17: 1346–1357, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

