The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA
- PMID: 29752462
- PMCID: PMC6008101
- DOI: 10.1038/s41584-018-0010-z
The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA
Abstract
Currently, no disease-modifying osteoarthritis drugs (DMOADs) have been approved. Past clinical trials have failed for several reasons, including the commonly applied definition of eligibility based on radiographic assessment of joint structure. In the context of precision medicine, finding the appropriate patient for a specific treatment approach will be of increasing relevance. Phenotypic stratification by use of imaging at the time of determining eligibility for clinical trials will be paramount and cannot be achieved using radiography alone. Furthermore, identification of joints at high risk of rapid progression of osteoarthritis is needed in order to enable a more efficient DMOAD trial design. In addition, joints at high risk of collapse need to be excluded at screening. The use of MRI might offer advantages over radiography in this context. Technological advances and simplified image assessment address many of the commonly perceived barriers to the application of MRI to assessment of eligibility for DMOAD clinical trials.
Figures

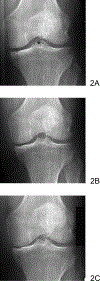

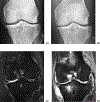
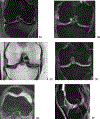
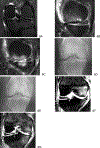
References
-
- Karsdal MA, et al. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthritis Cartilage. 2010;18:150–9. - PubMed
-
- Alexandersen P, Karsdal MA, Byrjalsen I, Christiansen C. Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric. 2011;14:236–43. - PubMed
-
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

