Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions
- PMID: 29753208
- PMCID: PMC6006912
- DOI: 10.1016/j.redox.2018.04.019
Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions
Abstract
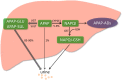
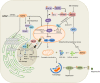
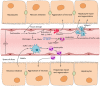
Acetaminophen (APAP) overdose is the leading cause of drug-induced acute liver failure in many developed countries. Mitochondrial oxidative stress is considered to be the predominant cellular event in APAP-induced liver injury. Accordingly, N-acetyl cysteine, a known scavenger of reactive oxygen species (ROS), is recommended as an effective clinical antidote against APAP-induced acute liver injury (AILI) when it is given at an early phase; however, the narrow therapeutic window limits its use. Hence, the development of novel therapeutic approaches that can offer broadly protective effects against AILI is clearly needed. To this end, it is necessary to better understand the mechanisms of APAP hepatotoxicity. Up to now, in addition to mitochondrial oxidative stress, many other cellular processes, including phase I/phase II metabolism, endoplasmic reticulum stress, autophagy, sterile inflammation, microcirculatory dysfunction, and liver regeneration, have been identified to be involved in the pathogenesis of AILI, providing new targets for developing more effective therapeutic interventions against APAP-induced liver injury. In this review, we summarize intracellular and extracellular events involved in APAP hepatotoxicity, along with emphatic discussions on the possible therapeutic approaches targeting these different cellular events.
Keywords: Acetaminophen; Autophagy; Endoplasmic reticulum stress; Liver regeneration; Mitochondrial oxidative stress; Sterile inflammation.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Ahmad N., Tahir M., Lone K.P. Amelioration of acetaminophen induced hepatotoxicity by methanolic extract of pomegranate peels in rats. J. Pak. Med. Assoc. 2016;66(7):859–863. - PubMed
-
- Ajiboye T.O. Lophirones B and C attenuate acetaminophen-induced liver damage in mice: studies on hepatic, oxidative stress and inflammatory biomarkers. J. Biochem. Mol. Toxicol. 2016;30(10):497–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

