Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment
- PMID: 29753658
- PMCID: PMC6064211
- DOI: 10.1016/j.antiviral.2018.05.001
Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment
Abstract
There is an urgent need for strategies to combat dengue virus (DENV) infection; a major global threat. We reported that the cellular kinases AAK1 and GAK regulate intracellular trafficking of multiple viruses and that sunitinib and erlotinib, approved anticancer drugs with potent activity against these kinases, protect DENV-infected mice from mortality. Nevertheless, further characterization of the therapeutic potential and underlying mechanism of this approach is required prior to clinical evaluation. Here, we demonstrate that sunitinib/erlotinib combination achieves sustained suppression of systemic infection at approved dose in DENV-infected IFN-α/β and IFN-γ receptor-deficient mice. Nevertheless, treatment with these blood-brain barrier impermeable drugs delays, yet does not prevent, late-onset paralysis; a common manifestation in this immunodeficient mouse model but not in humans. Sunitinib and erlotinib treatment also demonstrates efficacy in human primary monocyte-derived dendritic cells. Additionally, DENV infection induces expression of AAK1 transcripts, but not GAK, via single-cell transcriptomics, and these kinases are important molecular targets underlying the anti-DENV effect of sunitinib and erlotinib. Lastly, sunitinib/erlotinib combination alters inflammatory cytokine responses in DENV-infected mice. These findings support feasibility of repurposing sunitinib/erlotinib combination as a host-targeted antiviral approach and contribute to understanding its mechanism of antiviral action.
Keywords: Antivirals; Dengue virus; Drug repurposing; Kinase inhibitors; Virus-host interactions.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures


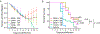
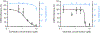

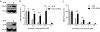

References
-
- Bekerman E, Neveu G, Shulla A, Brannan J, Pu S-Y, Wang S, Xiao F, Barouch-Bentov R, Bakken RR, Mateo R, Govero J, Nagamine CM, Diamond MS, De Jonghe S, Herdewijn P, Dye JM, Randall G, Einav S, 2017. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. The Journal of clinical investigation 127. - PMC - PubMed
-
- Bello CL, Sherman L, Zhou J, Verkh L, Smeraglia J, Mount J, Klamerus KJ, 2006. Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs 17, 353–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

