Obstructive Lymphangitis Precedes Colitis in Murine Norovirus-Infected Stat1-Deficient Mice
- PMID: 29753791
- PMCID: PMC6109697
- DOI: 10.1016/j.ajpath.2018.03.019
Obstructive Lymphangitis Precedes Colitis in Murine Norovirus-Infected Stat1-Deficient Mice
Abstract
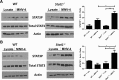
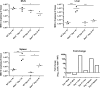
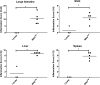
Murine norovirus (MNV) is an RNA virus that can prove lethal in mice with impaired innate immunity. We found that MNV-4 infection of Stat1-/- mice was not lethal, but produced a 100% penetrant, previously undescribed lymphatic phenotype characterized by chronic-active lymphangitis with hepatitis, splenitis, and chronic cecal and colonic inflammation. Lesion pathogenesis progressed from early ileal enteritis and regional dilated lymphatics to lymphangitis, granulomatous changes in the liver and spleen, and, ultimately, typhlocolitis. Lesion development was neither affected by antibiotics nor reproduced by infection with another enteric RNA virus, rotavirus. MNV-4 infection in Stat1-/- mice decreased expression of vascular endothelial growth factor (Vegf) receptor 3, Vegf-c, and Vegf-d and increased interferon (Ifn)-γ, tumor necrosis factor-α, and inducible nitric oxide synthase. However, anti-IFN-γ and anti-tumor necrosis factor-α antibody treatment did not attenuate the histologic lesions. Studies in Ifnαβγr-/- mice suggested that canonical signaling via interferon receptors did not cause MNV-4-induced disease. Infected Stat1-/- mice had increased STAT3 phosphorylation and expressed many STAT3-regulated genes, consistent with our findings of increased myeloid cell subsets and serum granulocyte colony-stimulating factor, which are also associated with increased STAT3 activity. In conclusion, in Stat1-/- mice, MNV-4 induces lymphatic lesions similar to those seen in Crohn disease as well as hepatitis, splenitis, and typhlocolitis. MNV-4-infected Stat1-/- mice may be a useful model to study mechanistic associations between viral infections, lymphatic dysfunction, and intestinal inflammation in a genetically susceptible host.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





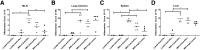


References
-
- Crohn B.B., Ginzburg L., Oppenheimer G.D. Regional ileitis a pathologic and clinical entity. J Am Med Assoc. 1932;99:1323–1329. - PubMed
-
- Alexander J.S., Chaitanya G.V., Grisham M.B., Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207 Suppl 1:E75–E85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

