Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways
- PMID: 29754206
- PMCID: PMC6015107
- DOI: 10.1007/s00401-018-1862-7
Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways
Abstract
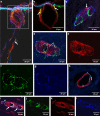
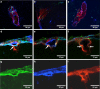
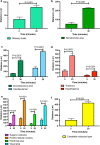
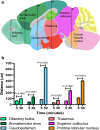
Tracers injected into CSF pass into the brain alongside arteries and out again. This has been recently termed the "glymphatic system" that proposes tracers enter the brain along periarterial "spaces" and leave the brain along the walls of veins. The object of the present study is to test the hypothesis that: (1) tracers from the CSF enter the cerebral cortex along pial-glial basement membranes as there are no perivascular "spaces" around cortical arteries, (2) tracers leave the brain along smooth muscle cell basement membranes that form the Intramural Peri-Arterial Drainage (IPAD) pathways for the elimination of interstitial fluid and solutes from the brain. 2 μL of 100 μM soluble, fluorescent fixable amyloid β (Aβ) were injected into the CSF of the cisterna magna of 6-10 and 24-30 month-old male mice and their brains were examined 5 and 30 min later. At 5 min, immunocytochemistry and confocal microscopy revealed Aβ on the outer aspects of cortical arteries colocalized with α-2 laminin in the pial-glial basement membranes. At 30 min, Aβ was colocalised with collagen IV in smooth muscle cell basement membranes in the walls of cortical arteries corresponding to the IPAD pathways. No evidence for drainage along the walls of veins was found. Measurements of the depth of penetration of tracer were taken from 11 regions of the brain. Maximum depths of penetration of tracer into the brain were achieved in the pons and caudoputamen. Conclusions drawn from the present study are that tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. The exit route is along IPAD pathways in which Aβ accumulates in cerebral amyloid angiopathy (CAA) in Alzheimer's disease. Results from this study suggest that CSF may be a suitable route for delivery of therapies for neurological diseases, including CAA.
Keywords: Basement membranes; Cerebrospinal fluid; Glymphatic; Interstitial fluid; Intramural periarterial drainage.
Figures


References
-
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. - DOI - PubMed
-
- Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol. 2013;39:593–611. doi: 10.1111/nan.12042. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

