Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology
- PMID: 29754415
- PMCID: PMC6033183
- DOI: 10.1002/1873-3468.13090
Biological hydropersulfides and related polysulfides - a new concept and perspective in redox biology
Abstract
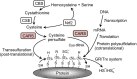
The chemical biology of thiols (RSH, e.g., cysteine and cysteine-containing proteins/peptides) has been a topic of extreme interest for many decades due to their reported roles in protein structure/folding, redox signaling, metal ligation, cellular protection, and enzymology. While many of the studies on thiol/sulfur biochemistry have focused on thiols, relatively ignored have been hydropersulfides (RSSH) and higher order polysulfur species (RSSn H, RSSn R, n > 1). Recent and provocative work has alluded to the prevalence and likely physiological importance of RSSH and related RSSn H. RSSH of cysteine (Cys-SSH) has been found to be prevalent in mammalian systems along with Cys-SSH-containing proteins. The RSSH functionality has not been examined to the extent of other biologically relevant sulfur derivatives (e.g., sulfenic acids, disulfides, etc.), whose roles in cell signaling are strongly indicated. The recent finding of Cys-SSH biosynthesis and translational incorporation into proteins is an unequivocal indication of its fundamental importance and necessitates a more profound look into the physiology of RSSH. In this Review, we discuss the currently reported chemical biology of RSSH (and related species) as a prelude to discussing their possible physiological roles.
Keywords: cysteine persulfides; hydropersulfides; translational polysulfidation.
© 2018 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures


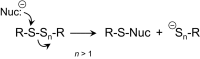
References
-
- Winterbourn CC and Hampton MB (2008) Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45, 549–561. - PubMed
-
- Reddie KG and Carroll KS (2008) Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol 12, 746–754. - PubMed
-
- Jacob C, Giles GI, Giles NM and Sies H (2003) Sulfur and Selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed 42, 4742–4758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

