Exploration of Near-Infrared-Emissive Colloidal Multinary Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform
- PMID: 29754493
- PMCID: PMC6024237
- DOI: 10.1021/acsnano.8b01122
Exploration of Near-Infrared-Emissive Colloidal Multinary Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform
Abstract
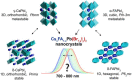
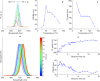
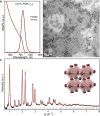
Hybrid organic-inorganic and fully inorganic lead halide perovskite nanocrystals (NCs) have recently emerged as versatile solution-processable light-emitting and light-harvesting optoelectronic materials. A particularly difficult challenge lies in warranting the practical utility of such semiconductor NCs in the red and infrared spectral regions. In this context, all three archetypal A-site monocationic perovskites-CH3NH3PbI3, CH(NH2)2PbI3, and CsPbI3-suffer from either chemical or thermodynamic instabilities in their bulk form. A promising approach toward the mitigation of these challenges lies in the formation of multinary compositions (mixed cation and mixed anion). In the case of multinary colloidal NCs, such as quinary Cs xFA1- xPb(Br1- yI y)3 NCs, the outcome of the synthesis is defined by a complex interplay between the bulk thermodynamics of the solid solutions, crystal surface energies, energetics, dynamics of capping ligands, and the multiple effects of the reagents in solution. Accordingly, the rational synthesis of such NCs is a formidable challenge. Herein, we show that droplet-based microfluidics can successfully tackle this problem and synthesize Cs xFA1- xPbI3 and Cs xFA1- xPb(Br1- yI y)3 NCs in both a time- and cost-efficient manner. Rapid in situ photoluminescence and absorption measurements allow for thorough parametric screening, thereby permitting precise optical engineering of these NCs. In this showcase study, we fine-tune the photoluminescence maxima of such multinary NCs between 700 and 800 nm, minimize their emission line widths (to below 40 nm), and maximize their photoluminescence quantum efficiencies (up to 89%) and phase/chemical stabilities. Detailed structural analysis revealed that the Cs xFA1- xPb(Br1- yI y)3 NCs adopt a cubic perovskite structure of FAPbI3, with iodide anions partially substituted by bromide ions. Most importantly, we demonstrate the excellent transference of reaction parameters from microfluidics to a conventional flask-based environment, thereby enabling up-scaling and further implementation in optoelectronic devices. As an example, Cs xFA1- xPb(Br1- yI y)3 NCs with an emission maximum at 735 nm were integrated into light-emitting diodes, exhibiting a high external quantum efficiency of 5.9% and a very narrow electroluminescence spectral bandwidth of 27 nm.
Keywords: formamidinium; halides; microfluidics; nanocrystals; perovskites; quantum dots.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Dismantling the "Red Wall" of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium-Cesium Lead Iodide Nanocrystals.ACS Nano. 2017 Mar 28;11(3):3119-3134. doi: 10.1021/acsnano.7b00116. Epub 2017 Mar 3. ACS Nano. 2017. PMID: 28231432 Free PMC article.
-
Unveiling the Shape Evolution and Halide-Ion-Segregation in Blue-Emitting Formamidinium Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform.Nano Lett. 2018 Feb 14;18(2):1246-1252. doi: 10.1021/acs.nanolett.7b04838. Epub 2018 Jan 19. Nano Lett. 2018. PMID: 29337579
-
Synthesis of Cesium Lead Halide Perovskite Nanocrystals in a Droplet-Based Microfluidic Platform: Fast Parametric Space Mapping.Nano Lett. 2016 Mar 9;16(3):1869-77. doi: 10.1021/acs.nanolett.5b04981. Epub 2016 Feb 4. Nano Lett. 2016. PMID: 26836149
-
Colloidal Metal-Halide Perovskite Nanoplatelets: Thickness-Controlled Synthesis, Properties, and Application in Light-Emitting Diodes.Adv Mater. 2022 Mar;34(10):e2107105. doi: 10.1002/adma.202107105. Epub 2022 Jan 28. Adv Mater. 2022. PMID: 34775643 Review.
-
Doping and ion substitution in colloidal metal halide perovskite nanocrystals.Chem Soc Rev. 2020 Jul 21;49(14):4953-5007. doi: 10.1039/c9cs00790c. Chem Soc Rev. 2020. PMID: 32538382 Review.
Cited by
-
Effect of polymer concentration on the morphology of the PHPMAA-g-PLA graft copolymer nanoparticles produced by microfluidics nanoprecipitation.Nanoscale Adv. 2024 Mar 11;6(8):1992-1996. doi: 10.1039/d3na01038d. eCollection 2024 Apr 16. Nanoscale Adv. 2024. PMID: 38633038 Free PMC article.
-
Microfluidics-enabled intelligent manufacturing of metal halide perovskite nanocrystals.Biomicrofluidics. 2023 Dec 12;17(6):061304. doi: 10.1063/5.0172135. eCollection 2023 Dec. Biomicrofluidics. 2023. PMID: 38094714 Free PMC article.
-
Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes.ACS Energy Lett. 2019 Nov 8;4(11):2703-2711. doi: 10.1021/acsenergylett.9b01915. Epub 2019 Oct 11. ACS Energy Lett. 2019. PMID: 31737780 Free PMC article.
-
Colloidal Aziridinium Lead Bromide Quantum Dots.ACS Nano. 2024 Feb 6;18(7):5684-97. doi: 10.1021/acsnano.3c11579. Online ahead of print. ACS Nano. 2024. PMID: 38320982 Free PMC article.
-
Perovskite Quantum Dots for Emerging Displays: Recent Progress and Perspectives.Nanomaterials (Basel). 2022 Jun 29;12(13):2243. doi: 10.3390/nano12132243. Nanomaterials (Basel). 2022. PMID: 35808081 Free PMC article. Review.
References
-
- Akkerman Q. A.; Gandini M.; Di Stasio F.; Rastogi P.; Palazon F.; Bertoni G.; Ball J. M.; Prato M.; Petrozza A.; Manna L. Strongly Emissive Perovskite Nanocrystal Inks for High-Voltage Solar Cells. Nat. Energy 2016, 2, 16194.10.1038/nenergy.2016.194. - DOI
-
- Kim H. S.; Lee C. R.; Im J. H.; Lee K. B.; Moehl T.; Marchioro A.; Moon S. J.; Humphry-Baker R.; Yum J. H.; Moser J. E.; Gratzel M.; Park N. G. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591.10.1038/srep00591. - DOI - PMC - PubMed
-
- Saliba M.; Matsui T.; Domanski K.; Seo J. Y.; Ummadisingu A.; Zakeeruddin S. M.; Correa-Baena J. P.; Tress W. R.; Abate A.; Hagfeldt A.; Gratzel M. Incorporation of Rubidium Cations into Perovskite Solar Cells Improves Photovoltaic Performance. Science 2016, 354, 206–209. 10.1126/science.aah5557. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

