Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design
- PMID: 29754661
- PMCID: PMC6517320
- DOI: 10.1016/j.ahj.2018.02.015
Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design
Abstract
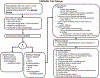
The Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA,NCT00911508)(1) trial is testing the hypothesis that the treatment strategy of percutaneous left atrial catheter ablation for the purpose of eliminating atrial fibrillation (AF) is superior to current state-of-the-art pharmacologic therapy. This international 140-center clinical trial was designed to randomize 2200 patients to a strategy of catheter ablation versus state-of-the-art rate or rhythm control drug therapy. Inclusion criteria include: 1) age> 65, or ≤65 with≥ 1 risk factor for stroke, 2) documented AF warranting treatment, and 3) eligibility for both catheter ablation and≥ 2 anti-arrhythmic or≥ 2 rate control drugs. Patients were followed every 3 to 6 months (median 4 years) and underwent repeat trans-telephonic monitoring, Holter monitoring, and CT/MR in a subgroup of patient studies to assess the impact of treatment on AF recurrence and atrial structure. With 1100 patients in each treatment arm, CABANA is projected to have 90% power for detecting a 30% relative reduction in the primary composite endpoint of total mortality, disabling stroke, serious bleeding, or cardiac arrest. Secondary endpoints include total mortality; mortality or cardiovascular hospitalization; a combination of mortality, stroke, hospitalization for heart failure or acute coronary artery events; cardiovascular death alone; and heart failure death, as well as AF recurrence, quality of life, and cost effectiveness. At a time when AF incidence is rising rapidly, CABANA will provide critical evidence with which to guide therapy and shape health care policy related to AF for years to come.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
CABANA trial: disappointing results?Eur Heart J Cardiovasc Pharmacother. 2019 Jan 1;5(1):57. doi: 10.1093/ehjcvp/pvy045. Eur Heart J Cardiovasc Pharmacother. 2019. PMID: 30496381 No abstract available.
References
-
- Go A, Hylek E, Phillip K, et al. Prevalence of diagnosed AF in adults. Natonal implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in AF (ATRIA). JAMA 2001;285:2370–5. - PubMed
-
- Lloyd-Jones D, Wang T, Leip E, et al. Lifetime risk for development of AF. The Framingham Heart Study. Circulation 2004;110:1042–6. - PubMed
-
- Greenlee R, Vidaillet H. Recent progress in the epidemiology of atrial fibrillation. Curr Opin Cardiol 2005;20:7–14. - PubMed
-
- Maisel W, Stevenson L. AF in heart failure: epidemiology, pathophysiology, and rantionale for therapy. AmJ Cardiol 2003;91:2D–8D. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

