Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study
- PMID: 29755117
- PMCID: PMC6162236
- DOI: 10.1038/s41416-018-0100-3
Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study
Abstract
Background: To assess the safety profile, pharmacokinetics, pharmacodynamics and preliminary antitumour activity of fixed-dose SHR-1210, a novel anti-PD-1 antibody, in advanced solid tumours.
Methods: A total of 36 patients with advanced solid tumours received intravenous SHR-1210 at 60 mg, 200 mg and 400 mg (4-week interval after first dose followed by a 2-week schedule) until disease progression or intolerable toxicity. The concentration of SHR-1210 was detected for pharmacokinetics, and receptor occupancy on circulating T lymphocytes was assessed for pharmacodynamics.
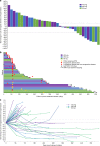
Results: No dose-limiting toxicities were observed. Maximum administered dose was not reached. Most adverse events were grade 1 or 2. Treatment-related severe adverse events were found in two patients. No treatment-related death was reported. Two complete responses (gastric cancer, bladder carcinoma) and seven partial responses were seen. In responders, the median follow-up time was 16.0 months (range 8.3-19.5), and the median duration of response was not reached (range 2.7-17.5+ months). The half-life of SHR-1210 was 2.94 d, 5.61 d and 11.0 d for 3 dose levels, respectively.
Conclusions: Our results demonstrated a promising antitumour activity and a manageable safety profile of SHR-1210, displayed an explicit PK evidence of the feasibility of fixed dose, and established the foundation for further exploration.
Conflict of interest statement
Q.Y. is a salaried employee of Jiangsu Hengrui Medicine Co. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

