THE LOCAL INFLUENCE OF PIONEER INVESTIGATORS ON TECHNOLOGY ADOPTION: EVIDENCE FROM NEW CANCER DRUGS
- PMID: 29755142
- PMCID: PMC5947964
- DOI: 10.1162/REST_a_00670
THE LOCAL INFLUENCE OF PIONEER INVESTIGATORS ON TECHNOLOGY ADOPTION: EVIDENCE FROM NEW CANCER DRUGS
Abstract
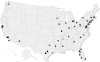
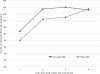
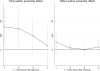
Local opinion leaders may play a key role in easing information frictions associated with technology adoption. This paper analyzes the influence of physician investigators who lead clinical trials for new cancer drugs. By comparing diffusion patterns across 21 new cancer drugs, we separate correlated regional demand for new technology from information spillovers. Patients in the lead investigator's region are initially 36% more likely to receive the new drug, but utilization converges within four years. We also find that superstar physician authors, measured by trial role or citation history, have broader influence than less prominent authors.
Figures
References
-
- Audretsch David B, Feldman Maryann P. Knowledge Spillovers and the Geography of Innovation. In: Henderson J Vernon, Thisse Jacques-François., editors. Handbook of Regional and Urban Economics. Vol. 4. Amsterdam: Elsevier; 2004. pp. 2713–2739.
-
- Azoulay Pierre, Graff Zivin Joshua S, Sampat Bhaven N. The Diffusion of Scientific Knowledge across Time and Space: Evidence from Professional Transitions for the Superstars of Medicine. Chicago: University of Chicago Press; 2012.
-
- Bach Peter B. Limits on Medicare’s Ability to Control Rising Spending on Cancer Drugs. New England Journal of Medicine. 2009;360:626–633. - PubMed
-
- Baerlocher Mark Otto, Newton Marshall, Gautam Tina, Tomlinson George, Detsky Allan S. The Meaning of Author Order in Medical Research. Journal of Investigative Medicine. 2007;55:174. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
