Remote C-H Functionalization via Selective Hydrogen Atom Transfer
- PMID: 29755145
- PMCID: PMC5940016
- DOI: 10.1055/s-0036-1591930
Remote C-H Functionalization via Selective Hydrogen Atom Transfer
Abstract
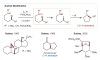
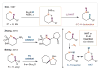
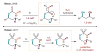
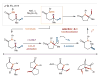
The selective functionalization of remote C-H bonds via intramolecular hydrogen atom transfer (HAT) is transformative for organic synthesis. This radical-mediated strategy provides access to novel reactivity that is complementary to closed-shell pathways. As modern methods for mild generation of radicals are continually developed, inherent selectivity paradigms of HAT mechanisms offer unparalleled opportunities for developing new strategies for C-H functionalization. This review outlines the history, recent advances, and mechanistic underpinnings of intramolecular HAT as a guide to addressing ongoing challenges in this arena.
Keywords: hydrogen atom transfer; nitrogen-centered radicals; oxygen-centered radicals; radicals; remote C–H functionalization.
Figures





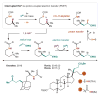



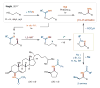
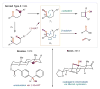
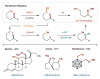

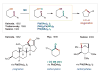
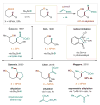
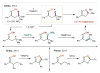



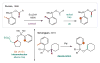






References
-
- Bergman RG. Nature (London) 2007;446:391. - PubMed
-
- White MC. Science (Washington, D C) 2012;335:807. - PubMed
-
- Yamaguchi J, Yamaguchi AD, Itami K. Angew Chem Int Ed. 2012;51:8960. - PubMed
-
- Wencel-Delord J, Glorius F. Nat Chem. 2013;5:369. - PubMed
-
- Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem Soc Rev. 2009;38:3242. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
