Inhibition of the PI3K but not the MEK/ERK pathway sensitizes human glioma cells to alkylating drugs
- PMID: 29755294
- PMCID: PMC5935937
- DOI: 10.1186/s12935-018-0565-4
Inhibition of the PI3K but not the MEK/ERK pathway sensitizes human glioma cells to alkylating drugs
Abstract
Background: Intrinsic chemoresistance of glioblastoma (GBM) is frequently owed to activation of the PI3K and MEK/ERK pathways. These signaling cascades are tightly interconnected however the quantitative contribution of both to intrinsic resistance is still not clear. Here, we aimed at determining the activation status of these pathways in human GBM biopsies and cells and investigating the quantitative impact of both pathways to chemoresistance.
Methods: Receptor tyrosine kinase (RTK) pathways in temozolomide (TMZ) treatment naive or TMZ resistant human GBM biopsies and GBM cells were investigated by proteome profiling and immunoblotting of a subset of proteins. Resistance to drugs and RTK pathway inhibitors was assessed by MTT assays. Apoptotic rates were determined by Annexin V staining and DNA damage with comet assays and immunoblotting.
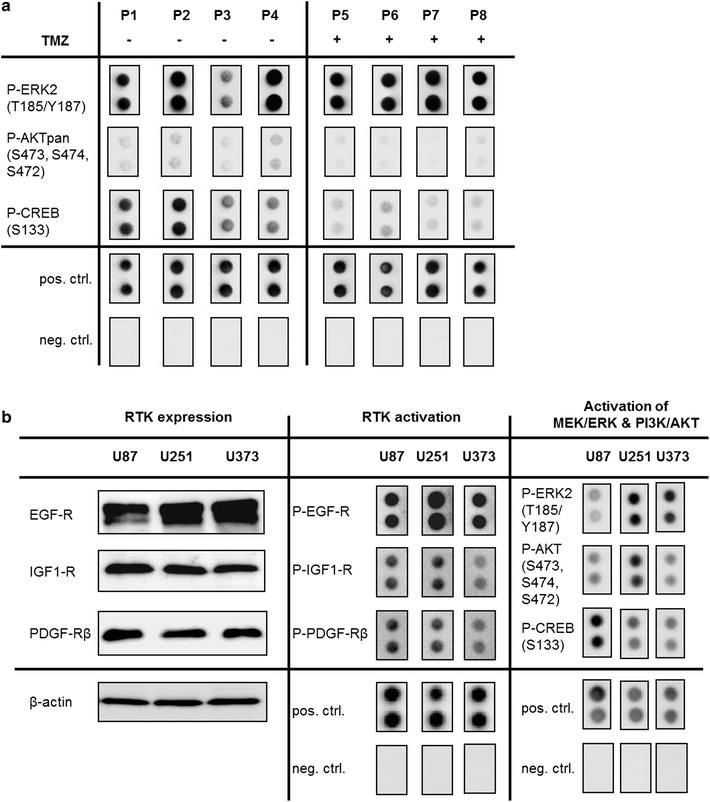
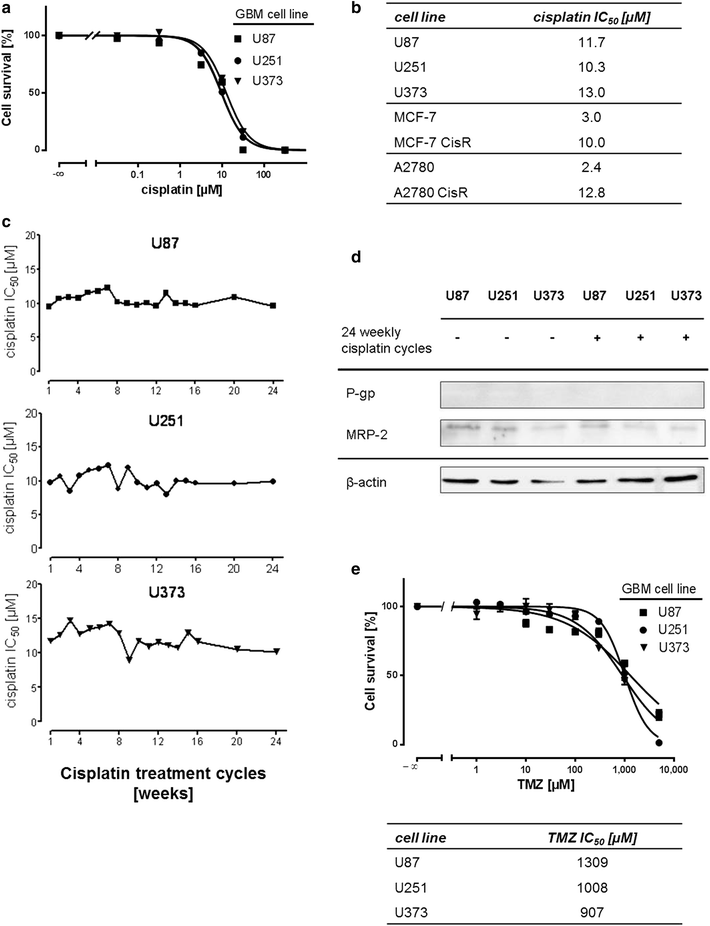
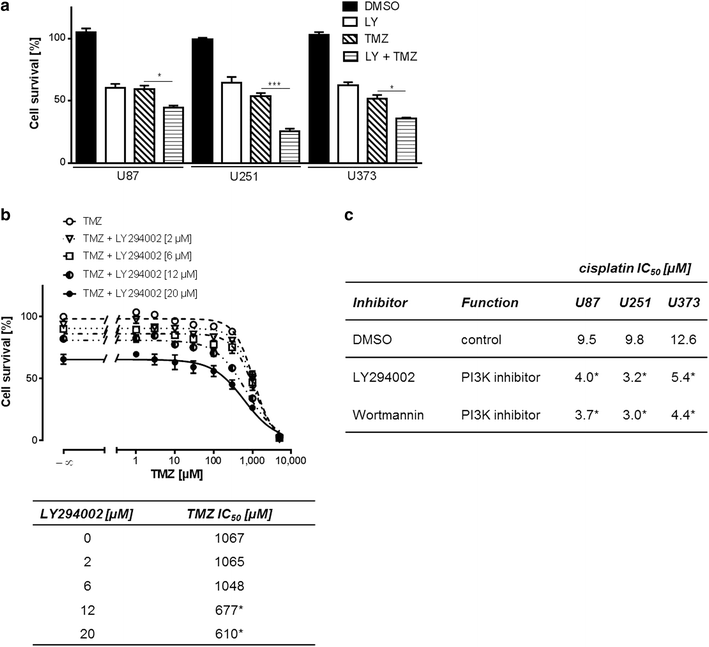
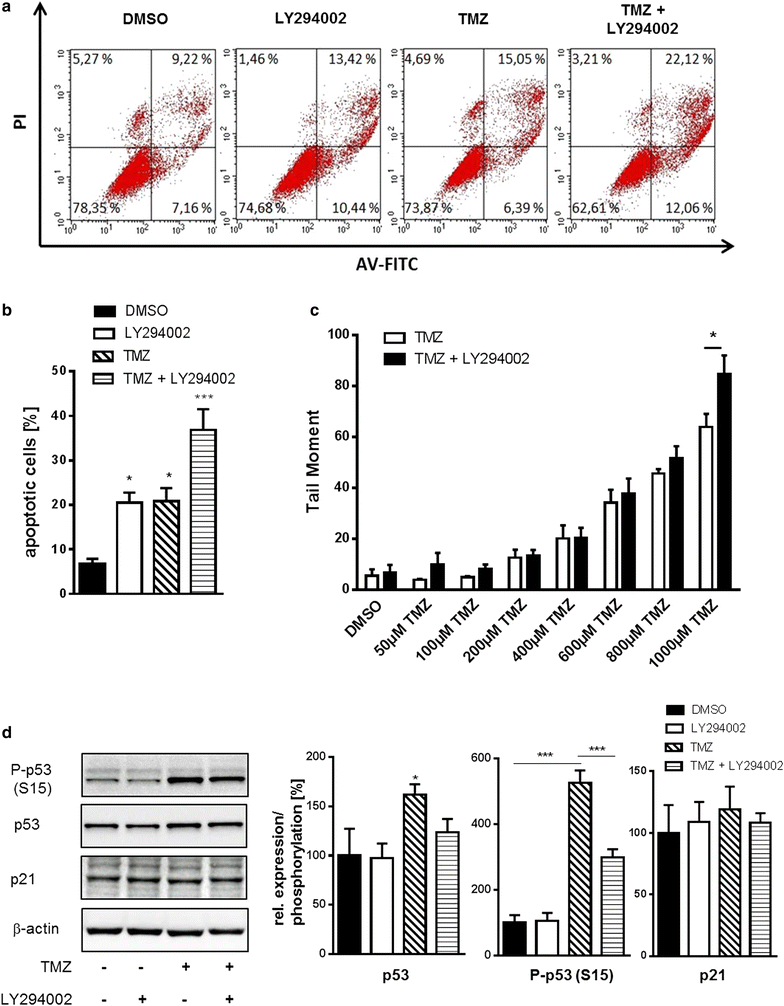
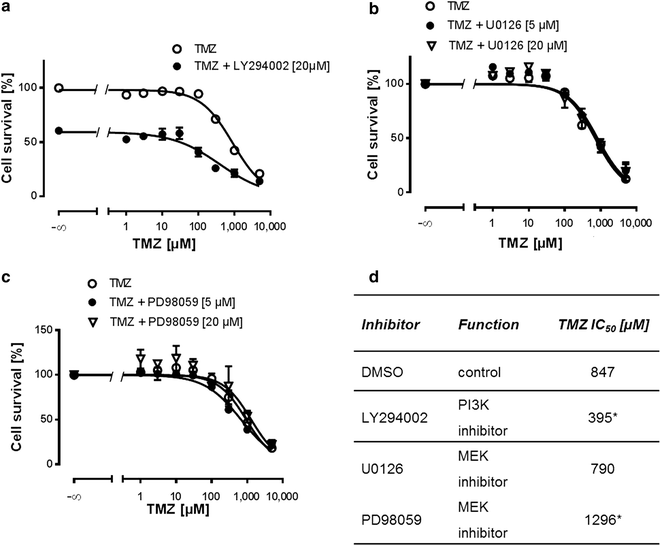
Results: We analyzed activation of RTK pathways by proteome profiling of tumor samples of patients which were diagnosed a secondary GBM and underwent surgery and patients which underwent a second surgery after TMZ treatment due to recurrence of the tumor. We observed substantial activation of the PI3K and MEK/ERK pathways in both groups. However, AKT and CREB phosphorylation was reduced in biopsies of resistant tumors while ERK phosphorylation remained unchanged. Subsequent proteome profiling revealed that multiple RTKs and downstream targets are also activated in three GBM cell lines. We then systematically describe a mechanism of resistance of GBM cell lines and human primary GBM cells to the alkylating drugs TMZ and cisplatin. No specific inhibitor of the upstream RTKs sensitized cells to drug treatment. In contrast, we were able to restore sensitivity to TMZ and cisplatin by inhibiting PI3K in all cell lines and in human primary GBM cells. Interestingly, an opposite effect was observed when we inhibited the MEK/ERK signaling cascade with two different inhibitors.
Conclusions: Temozolomide treatment naive and TMZ resistant GBM biopsies show a distinct activation pattern of the MEK/ERK and PI3K signaling cascades indicating a role of these pathways in resistance development. Both pathways are also activated in GBM cell lines, however, only the PI3K pathway seems to play a crucial role in resistance to alkylating agents and might serve as drug target for chemosensitization.
Keywords: Cisplatin; Drug resistance; Glioblastoma; PI3K; Temozolomide.
Figures

References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

