Arc 3' UTR Splicing Leads to Dual and Antagonistic Effects in Fine-Tuning Arc Expression Upon BDNF Signaling
- PMID: 29755318
- PMCID: PMC5934489
- DOI: 10.3389/fnmol.2018.00145
Arc 3' UTR Splicing Leads to Dual and Antagonistic Effects in Fine-Tuning Arc Expression Upon BDNF Signaling
Abstract
Activity-regulated cytoskeletal associated protein (Arc) is an immediate-early gene critically involved in synaptic plasticity and memory consolidation. Arc mRNA is rapidly induced by synaptic activation and a portion is locally translated in dendrites where it modulates synaptic strength. Being an activity-dependent effector of homeostatic balance, regulation of Arc is uniquely tuned to result in short-lived bursts of expression. Cis-Acting elements that control its transitory expression post-transcriptionally reside primarily in Arc mRNA 3' UTR. These include two conserved introns which distinctively modulate Arc mRNA stability by targeting it for destruction via the nonsense mediated decay pathway. Here, we further investigated how splicing of the Arc mRNA 3' UTR region contributes to modulate Arc expression in cultured neurons. Unexpectedly, upon induction with brain derived neurotrophic factor, translational efficiency of a luciferase reporter construct harboring Arc 3' UTR is significantly upregulated and this effect is dependent on splicing of Arc introns. We find that, eIF2α dephosphorylation, mTOR, ERK, PKC, and PKA activity are key to this process. Additionally, CREB-dependent transcription is required to couple Arc 3' UTR-splicing to its translational upregulation, suggesting the involvement of de novo transcribed trans-acting factors. Overall, splicing of Arc 3' UTR exerts a dual and unique effect in fine-tuning Arc expression upon synaptic signaling: while inducing mRNA decay to limit the time window of Arc expression, it also elicits translation of the decaying mRNA. This antagonistic effect likely contributes to the achievement of a confined yet efficient burst of Arc protein expression, facilitating its role as an effector of synapse-specific plasticity.
Keywords: 3′ UTR; Arc; BDNF; EJC; nonsense mediated mRNA decay; plasticity; post-transcriptional regulation; splicing.
Figures

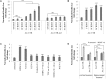


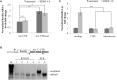
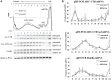
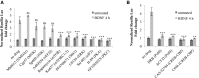
References
-
- Aicardi G., Argilli E., Cappello S., Santi S., Riccio M., Thoenen H., et al. (2004). Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 101 15788–15792. 10.1073/pnas.0406960101 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

