Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation
- PMID: 29755458
- PMCID: PMC5932189
- DOI: 10.3389/fimmu.2018.00804
Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation
Abstract
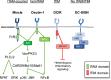
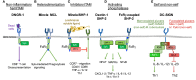
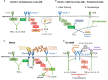
Myeloid C-type lectin receptors (CLRs) are important sensors of self and non-self that work in concert with other pattern recognition receptors (PRRs). CLRs have been previously classified based on their signaling motifs as activating or inhibitory receptors. However, specific features of the ligand binding process may result in distinct signaling through a single motif, resulting in the triggering of non-canonical pathways. In addition, CLR ligands are frequently exposed in complex structures that simultaneously bind different CLRs and other PRRs, which lead to integration of heterologous signaling among diverse receptors. Herein, we will review how sensing by myeloid CLRs and crosstalk with heterologous receptors is modulated by many factors affecting their signaling and resulting in differential outcomes for immunity and inflammation. Finding common features among those flexible responses initiated by diverse CLR-ligand partners will help to harness CLR function in immunity and inflammation.
Keywords: dendritic cells; inflammation; innate immunity; lectin receptors; macrophages; monocytes; signaling.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

