Comparison of Phenotypic and Functional Characteristics Between Canine Non-B, Non-T Natural Killer Lymphocytes and CD3+CD5dimCD21- Cytotoxic Large Granular Lymphocytes
- PMID: 29755462
- PMCID: PMC5934500
- DOI: 10.3389/fimmu.2018.00841
Comparison of Phenotypic and Functional Characteristics Between Canine Non-B, Non-T Natural Killer Lymphocytes and CD3+CD5dimCD21- Cytotoxic Large Granular Lymphocytes
Abstract
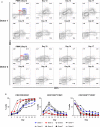
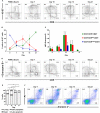
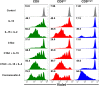
Natural killer (NK) cells play a pivotal role in the immune response against infections and malignant transformation, and adopted transfer of NK cells is thought to be a promising therapeutic approach for cancer patients. Previous reports describing the phenotypic features of canine NK cells have produced inconsistent results. Canine NK cells are still defined as non-B and non-T (CD3-CD21-) large granular lymphocytes. However, a few reports have demonstrated that canine NK cells share the phenotypic characteristics of T lymphocytes, and that CD3+CD5dimCD21- lymphocytes are putative canine NK cells. Based on our previous reports, we hypothesized that phenotypic modulation could occur between these two populations during activation. In this study, we investigated the phenotypic and functional differences between CD3+CD5dimCD21- (cytotoxic large granular lymphocytes) and CD3-CD5-CD21- NK lymphocytes before and after culture of peripheral blood mononuclear cells isolated from normal dogs. The results of this study show that CD3+CD5dimCD21- lymphocytes can be differentiated into non-B, non-T NK (CD3-CD5-CD21-TCRαβ-TCRγδ-GranzymeB+) lymphocytes through phenotypic modulation in response to cytokine stimulation. In vitro studies of purified CD3+CD5dimCD21- cells showed that CD3-CD5-CD21- cells are derived from CD3+CD5dimCD21- cells through phenotypic modulation. CD3+CD5dimCD21- cells share more NK cell functional characteristics compared with CD3-CD5-CD21- cells, including the expression of T-box transcription factors (Eomes, T-bet), the production of granzyme B and interferon-γ, and the expression of NK cell-related molecular receptors such as NKG2D and NKp30. In conclusion, the results of this study suggest that CD3+CD5dimCD21- and CD3-CD5-CD21- cells both contain a subset of putative NK cells, and the difference between the two populations may be due to the degree of maturation.
Keywords: canine; cytotoxic large granular lymphocytes; natural killer cells; non-B non-T lymphocytes; phenotypic modulation.
Figures



Similar articles
-
Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells.Vet Immunol Immunopathol. 2013 Jun 15;153(3-4):249-59. doi: 10.1016/j.vetimm.2013.03.006. Epub 2013 Mar 21. Vet Immunol Immunopathol. 2013. PMID: 23548866 Free PMC article.
-
Interleukin-21 induces proliferation and modulates receptor expression and effector function in canine natural killer cells.Vet Immunol Immunopathol. 2015 May 15;165(1-2):22-33. doi: 10.1016/j.vetimm.2015.03.004. Epub 2015 Mar 13. Vet Immunol Immunopathol. 2015. PMID: 25819349
-
NCR1 is an activating receptor expressed on a subset of canine NK cells.Vet Immunol Immunopathol. 2016 Sep;177:7-15. doi: 10.1016/j.vetimm.2016.05.001. Epub 2016 May 24. Vet Immunol Immunopathol. 2016. PMID: 27436439
-
Working in "NK Mode": Natural Killer Group 2 Member D and Natural Cytotoxicity Receptors in Stress-Surveillance by γδ T Cells.Front Immunol. 2018 Apr 24;9:851. doi: 10.3389/fimmu.2018.00851. eCollection 2018. Front Immunol. 2018. PMID: 29740448 Free PMC article. Review.
-
Expression of cytotoxic proteins in peripheral T-cell and natural killer-cell (NK) lymphomas: association with extranodal site, NK or Tgammadelta phenotype, anaplastic morphology and CD30 expression.Leuk Lymphoma. 2000 Jul;38(3-4):317-26. doi: 10.3109/10428190009087022. Leuk Lymphoma. 2000. PMID: 10830738 Review.
Cited by
-
Diagnosis of canine B-cell chronic lymphoid leukemia with a CD21 negative phenotype using the LT21 clone CD21 antibody in flow cytometry: a case report.BMC Vet Res. 2024 Oct 26;20(1):490. doi: 10.1186/s12917-024-04335-x. BMC Vet Res. 2024. PMID: 39462364 Free PMC article.
-
Establishment of an in vitro erythroid differentiation system from canine peripheral blood mononuclear cells.Anim Cells Syst (Seoul). 2025 Apr 28;29(1):251-263. doi: 10.1080/19768354.2025.2492148. eCollection 2025. Anim Cells Syst (Seoul). 2025. PMID: 40304014 Free PMC article.
-
Approaches to Enhance Natural Killer Cell-Based Immunotherapy for Pediatric Solid Tumors.Cancers (Basel). 2021 Jun 4;13(11):2796. doi: 10.3390/cancers13112796. Cancers (Basel). 2021. PMID: 34199783 Free PMC article. Review.
-
Natural Killer and T Cell Infiltration in Canine Osteosarcoma: Clinical Implications and Translational Relevance.Front Vet Sci. 2021 Nov 16;8:771737. doi: 10.3389/fvets.2021.771737. eCollection 2021. Front Vet Sci. 2021. PMID: 34869744 Free PMC article. Review.
-
Distinct Features of Canine Non-conventional CD4-CD8α- Double-Negative TCRαβ+ vs. TCRγδ+ T Cells.Front Immunol. 2019 Nov 22;10:2748. doi: 10.3389/fimmu.2019.02748. eCollection 2019. Front Immunol. 2019. PMID: 31824515 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

