Differential Effect of Viable Versus Necrotic Neutrophils on Mycobacterium tuberculosis Growth and Cytokine Induction in Whole Blood
- PMID: 29755473
- PMCID: PMC5934482
- DOI: 10.3389/fimmu.2018.00903
Differential Effect of Viable Versus Necrotic Neutrophils on Mycobacterium tuberculosis Growth and Cytokine Induction in Whole Blood
Abstract
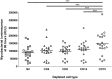
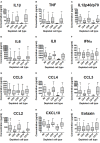
Neutrophils exert both positive and negative influences on the host response to tuberculosis, but the mechanisms by which these differential effects are mediated are unknown. We studied the impact of live and dead neutrophils on the control of Mycobacterium tuberculosis using a whole blood bioluminescence-based assay, and assayed supernatant cytokine concentrations using Luminex™ technology and ELISA. CD15+ granulocyte depletion from blood prior to infection with M. tuberculosis-lux impaired control of mycobacteria by 96 h, with a greater effect than depletion of CD4+, CD8+, or CD14+ cells (p < 0.001). Augmentation of blood with viable granulocytes significantly improved control of mycobacteria by 96 h (p = 0.001), but augmentation with necrotic granulocytes had the opposite effect (p = 0.01). Both augmentations decreased supernatant concentrations of tumor necrosis factor and interleukin (IL)-12 p40/p70, but necrotic granulocyte augmentation also increased concentrations of IL-10, G-CSF, GM-CSF, and CCL2. Necrotic neutrophil augmentation reduced phagocytosis of FITC-labeled M. bovis BCG by all phagocytes, whereas viable neutrophil augmentation specifically reduced early uptake by CD14+ cells. The immunosuppressive effect of dead neutrophils required necrotic debris rather than supernatant. We conclude that viable neutrophils enhance control of M. tuberculosis in blood, but necrotic neutrophils have the opposite effect-the latter associated with induction of IL-10, growth factors, and chemoattractants. Our findings suggest a mechanism by which necrotic neutrophils may exert detrimental effects on the host response in active tuberculosis.
Keywords: mycobacteria; necrosis; neutrophil; tuberculosis; viability.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

