Iron Retention in Root Hemicelluloses Causes Genotypic Variability in the Tolerance to Iron Deficiency-Induced Chlorosis in Maize
- PMID: 29755495
- PMCID: PMC5932200
- DOI: 10.3389/fpls.2018.00557
Iron Retention in Root Hemicelluloses Causes Genotypic Variability in the Tolerance to Iron Deficiency-Induced Chlorosis in Maize
Abstract
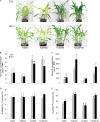
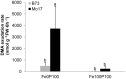
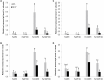
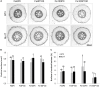
Antagonistic interactions of phosphorus (P) hamper iron (Fe) acquisition by plants and can cause Fe deficiency-induced chlorosis. To determine the physiological processes underlying adverse Fe-P interactions, the maize lines B73 and Mo17, which differ in chlorosis susceptibility, were grown hydroponically at different Fe:P ratios. In the presence of P, Mo17 became more chlorotic than B73. The higher sensitivity of Mo17 to Fe deficiency was not related to Fe-P interactions in leaves but to lower Fe translocation to shoots, which coincided with a larger pool of Fe being fixed in the root apoplast of P-supplied Mo17 plants. Fractionating cell wall components from roots showed that most of the cell wall-contained P accumulated in pectin, whereas most of the Fe was bound to root hemicelluloses, revealing that co-precipitation of Fe and P in the apoplast was not responsible for Fe inactivation in roots. A negative correlation between chlorophyll index and hemicellulose-bound Fe in 85 inbred lines of the intermated maize B73 × Mo17 (IBM) population indicated that apoplastic Fe retention contributes to genotypic differences in chlorosis susceptibility of maize grown under low Fe supplies. Our study indicates that Fe retention in the hemicellulose fraction of roots is an important determinant in the tolerance to Fe deficiency-induced chlorosis of graminaceous plant species with low phytosiderophore release, like maize.
Keywords: Fe deficiency; apoplastic iron; cell wall; iron–phosphorus interaction; strategy II.
Figures




References
-
- Ajakaiye C. O. (1979). Effect of phosphorus on growth and iron nutrition of millet and sorghum. Plant Soil 51 551–561. 10.1007/BF02277575 - DOI
-
- Bournier M., Tissot N., Mari S., Boucherez J., Lacombe E., Briat J. F. (2013). Arabidopsis ferritin 1 (AtFer1) gene regulation by the phosphate starvation response 1 (AtPHR1) transcription factor reveals a direct molecular link between iron and phosphate homeostasis. J. Biol. Chem. 288 22670–22680. 10.1074/jbc.M113.482281 - DOI - PMC - PubMed
-
- Carvalhais L. C., Dennis P. G., Fedoseyenko D., Hajirezaei M. R., Borriss R., von Wirén N. (2011). Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 174 3–11. 10.1002/jpln.201000085 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

