Enhanced Conjugation of Auxin by GH3 Enzymes Leads to Poor Adventitious Rooting in Carnation Stem Cuttings
- PMID: 29755501
- PMCID: PMC5932754
- DOI: 10.3389/fpls.2018.00566
Enhanced Conjugation of Auxin by GH3 Enzymes Leads to Poor Adventitious Rooting in Carnation Stem Cuttings
Abstract
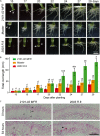
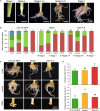
Commercial carnation (Dianthus caryophyllus) cultivars are vegetatively propagated from axillary stem cuttings through adventitious rooting; a process which is affected by complex interactions between nutrient and hormone levels and is strongly genotype-dependent. To deepen our understanding of the regulatory events controlling this process, we performed a comparative study of adventitious root (AR) formation in two carnation cultivars with contrasting rooting performance, "2101-02 MFR" and "2003 R 8", as well as in the reference cultivar "Master". We provided molecular evidence that localized auxin response in the stem cutting base was required for efficient adventitious rooting in this species, which was dynamically established by polar auxin transport from the leaves. In turn, the bad-rooting behavior of the "2003 R 8" cultivar was correlated with enhanced synthesis of indole-3-acetic acid conjugated to aspartic acid by GH3 proteins in the stem cutting base. Treatment of stem cuttings with a competitive inhibitor of GH3 enzyme activity significantly improved rooting of "2003 R 8". Our results allowed us to propose a working model where endogenous auxin homeostasis regulated by GH3 proteins accounts for the cultivar dependency of AR formation in carnation stem cuttings.
Keywords: Dianthus caryophyllus; IAA degradation; adventitious rooting; auxin homeostasis; auxin-conjugating enzymes; polar auxin transport; stem cuttings.
Figures





References
-
- Agulló-Antón M. A., Sánchez Bravo J., Acosta M., Druege U. (2011). Auxins or sugars: What makes the difference in the adventitious rooting of stored carnation cuttings? J. Plant Growth Regul. 30 100–113. 10.1007/s00344-010-9174-8 - DOI
-
- Agulló-Antón M. Á., Ferrández-Ayela A., Fernández-García N., Nicolás C., Albacete A., Pérez-Alfocea F., et al. (2014). Early steps of adventitious rooting: morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol. Plant. 150 446–462. 10.1111/ppl.12114 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

