Motor Improvement of Skilled Forelimb Use Induced by Treatment with Growth Hormone and Rehabilitation Is Dependent on the Onset of the Treatment after Cortical Ablation
- PMID: 29755514
- PMCID: PMC5883990
- DOI: 10.1155/2018/6125901
Motor Improvement of Skilled Forelimb Use Induced by Treatment with Growth Hormone and Rehabilitation Is Dependent on the Onset of the Treatment after Cortical Ablation
Abstract
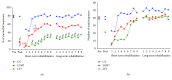
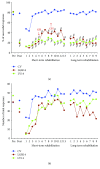
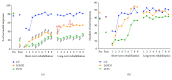
We previously demonstrated that the administration of GH immediately after severe motor cortex injury, in rats, followed by rehabilitation, improved the functionality of the affected limb and reexpressed nestin in the contralateral motor cortex. Here, we analyze whether these GH effects depend on a time window after the injury and on the reexpression of nestin and actin. Injured animals were treated with GH (0.15 mg/kg/day) or vehicle, at days 7, 14, and 35 after cortical ablation. Rehabilitation was applied at short and long term (LTR) after the lesion and then sacrificed. Nestin and actin were analyzed by immunoblotting in the contralateral motor cortex. Giving GH at days 7 or 35 after the lesion, but not 14 days after it, led to a remarkable improvement in the functionality of the affected paw. Contralateral nestin and actin reexpression was clearly higher in GH-treated animals, probably because compensatory brain plasticity was established. GH and immediate rehabilitation are key for repairing brain injuries, with the exception of a critical time period: GH treatment starting 14 days after the lesion. Our data also indicate that there is not a clear plateau in the recovery from a brain injury in agreement with our data in human patients.
Figures

Similar articles
-
Cell Proliferation in the Piriform Cortex of Rats with Motor Cortex Ablation Treated with Growth Hormone and Rehabilitation.Int J Mol Sci. 2021 May 21;22(11):5440. doi: 10.3390/ijms22115440. Int J Mol Sci. 2021. PMID: 34064044 Free PMC article.
-
Early growth hormone (GH) treatment promotes relevant motor functional improvement after severe frontal cortex lesion in adult rats.Behav Brain Res. 2013 Jun 15;247:48-58. doi: 10.1016/j.bbr.2013.03.012. Epub 2013 Mar 18. Behav Brain Res. 2013. PMID: 23518437
-
Factors Involved in the Functional Motor Recovery of Rats with Cortical Ablation after GH and Rehabilitation Treatment: Cortical Cell Proliferation and Nestin and Actin Expression in the Striatum and Thalamus.Int J Mol Sci. 2019 Nov 16;20(22):5770. doi: 10.3390/ijms20225770. Int J Mol Sci. 2019. PMID: 31744113 Free PMC article.
-
Attempt-dependent decrease in skilled reaching characterizes the acute postsurgical period following a forelimb motor cortex lesion: an experimental demonstration of learned nonuse in the rat.Behav Brain Res. 2007 May 16;179(2):208-18. doi: 10.1016/j.bbr.2007.02.004. Epub 2007 Feb 9. Behav Brain Res. 2007. PMID: 17346809
-
Recovery of motor function after stroke.Prog Brain Res. 2006;157:223-8. doi: 10.1016/S0079-6123(06)57015-3. Prog Brain Res. 2006. PMID: 17046674 Review.
Cited by
-
Cell Proliferation in the Piriform Cortex of Rats with Motor Cortex Ablation Treated with Growth Hormone and Rehabilitation.Int J Mol Sci. 2021 May 21;22(11):5440. doi: 10.3390/ijms22115440. Int J Mol Sci. 2021. PMID: 34064044 Free PMC article.
-
Growth Hormone Increases BDNF and mTOR Expression in Specific Brain Regions after Photothrombotic Stroke in Mice.Neural Plast. 2022 Apr 15;2022:9983042. doi: 10.1155/2022/9983042. eCollection 2022. Neural Plast. 2022. PMID: 35465399 Free PMC article.
-
Overexpression of kynurenic acid and 3-hydroxyanthranilic acid after rat traumatic brain injury.Eur J Histochem. 2018 Nov 14;62(4):2985. doi: 10.4081/ejh.2018.2985. Eur J Histochem. 2018. PMID: 30426733 Free PMC article.
-
Growth Hormone Promotes Motor Function after Experimental Stroke and Enhances Recovery-Promoting Mechanisms within the Peri-Infarct Area.Int J Mol Sci. 2020 Jan 17;21(2):606. doi: 10.3390/ijms21020606. Int J Mol Sci. 2020. PMID: 31963456 Free PMC article.
-
Growth Hormone and the Auditory Pathway: Neuromodulation and Neuroregeneration.Int J Mol Sci. 2021 Mar 11;22(6):2829. doi: 10.3390/ijms22062829. Int J Mol Sci. 2021. PMID: 33799503 Free PMC article. Review.
References
-
- Isgaard J., Aberg D., Nilsson M. Protective and regenerative effects of the GH/IGF-I axis on the brain. Minerva Endocrinologica. 2007;32(2):103–113. - PubMed
-
- Christophidis L. J., Gorba T., Gustavsson M., et al. Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: a potential role for growth hormone in injury-induced neurogenesis. Growth Hormone & IGF Research. 2009;19(6):497–506. doi: 10.1016/j.ghir.2009.05.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

