Evaluation of Diabetogenic Mechanism of High Fat Diet in Combination with Arsenic Exposure in Male Mice
- PMID: 29755549
- PMCID: PMC5937088
Evaluation of Diabetogenic Mechanism of High Fat Diet in Combination with Arsenic Exposure in Male Mice
Abstract
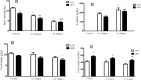
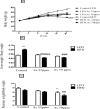
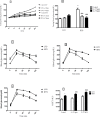
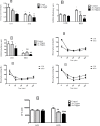
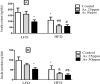
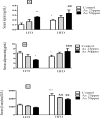
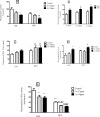
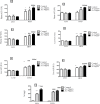
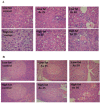
Obesity is a main reason of type 2 diabetes and also chronic exposure to arsenic (As) can produce diabetic symptoms. In previous studies, the association between high-fat diet and arsenic in the incidence of diabetes was found, but the role of beta cells activity, liver mitochondrial oxidative stress, and hepatic enzymes (leptin, adiponectin and beta amylase) was unclear. Thus, present study was conducted to evaluate the diabetogenic mechanism of arsenic followed by concomitant administration of high-fat diet (HFD) in male mice. In this experimental study, the mice consumed with HFD or low-fat diet (LFD) while exposed to As 25 or 50 ppm in drinking water for 20 weeks. At the end of experiments, hyperglycemia, insulin resistance variables, lipid profile, hepatic enzymes, liver mitochondrial oxidative stress, islet insulin secretion, liver, and pancreas histopathology were evaluated in all mice by their own methods. Control HFD fed mice showed a significant increase in FBG, OGTT, HOMA-IR, ITT, lipid profile, leptin, β-amylase, liver mitochondrial oxidative stress, hepatic enzymes and decreased FPI, HOMA-β, adiponectin, and islet insulin secretion or content. However, exposure to HFD concomitant with Arsenic revealed an impressive reduction in FBG, FPI, HOMA-IR, HOMA-β, ITT, lipid profile, and islet insulin secretion or content. This exposure enhanced OGTT, leptin, adiponectin, liver mitochondrial oxidative stress, and hepatic enzymes. In conclusion, HFD and arsenic concomitant administration induced impairment of OGTT and islet insulin secretion or content through the mitochondrial oxidative stress.
Keywords: Arsenic; High-fat diet; Islet insulin secretion; Mitochondrial oxidative stress; Type 2 diabetes.
Figures
References
-
- Wang W, Xie Z, Lin Y, Zhang D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J. Epidemiol. Community Health. 2014;68:176–84. - PubMed
-
- Wang A, Holladay SD, Wolf DC, Ahmed SA, Robertson JL. Reproductive and developmental toxicity of arsenic in rodents: A review. Int. J. Toxicol. 2006;25:319–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
