Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements
- PMID: 29755574
- PMCID: PMC5934885
- DOI: 10.1186/s12986-018-0271-1
Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements
Abstract
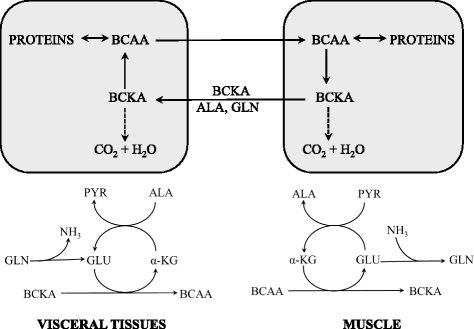
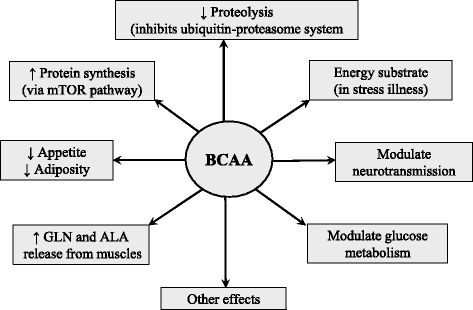
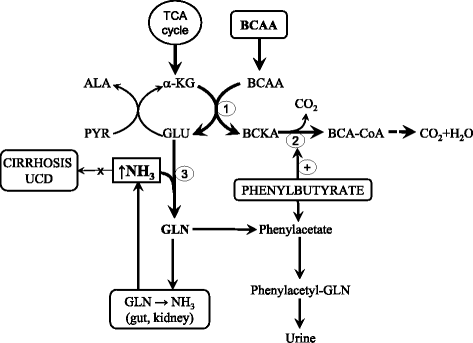
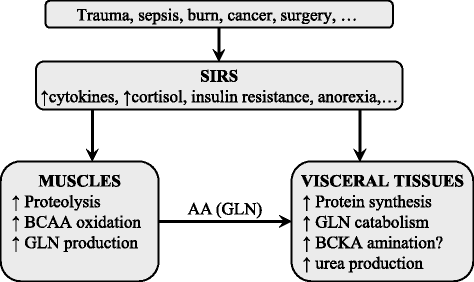
Branched-chain amino acids (BCAAs; valine, leucine, and isoleucine) are essential amino acids with protein anabolic properties, which have been studied in a number of muscle wasting disorders for more than 50 years. However, until today, there is no consensus regarding their therapeutic effectiveness. In the article is demonstrated that the crucial roles in BCAA metabolism play: (i) skeletal muscle as the initial site of BCAA catabolism accompanied with the release of alanine and glutamine to the blood; (ii) activity of branched-chain keto acid dehydrogenase (BCKD); and (iii) amination of branched-chain keto acids (BCKAs) to BCAAs. Enhanced consumption of BCAA for ammonia detoxification to glutamine in muscles is the cause of decreased BCAA levels in liver cirrhosis and urea cycle disorders. Increased BCKD activity is responsible for enhanced oxidation of BCAA in chronic renal failure, trauma, burn, sepsis, cancer, phenylbutyrate-treated subjects, and during exercise. Decreased BCKD activity is the main cause of increased BCAA levels and BCKAs in maple syrup urine disease, and plays a role in increased BCAA levels in diabetes type 2 and obesity. Increased BCAA concentrations during brief starvation and type 1 diabetes are explained by amination of BCKAs in visceral tissues and decreased uptake of BCAA by muscles. The studies indicate beneficial effects of BCAAs and BCKAs in therapy of chronic renal failure. New therapeutic strategies should be developed to enhance effectiveness and avoid adverse effects of BCAA on ammonia production in subjects with liver cirrhosis and urea cycle disorders. Further studies are needed to elucidate the effects of BCAA supplementation in burn, trauma, sepsis, cancer and exercise. Whether increased BCAA levels only markers are or also contribute to insulin resistance should be known before the decision is taken regarding their suitability in obese subjects and patients with type 2 diabetes. It is concluded that alterations in BCAA metabolism have been found common in a number of disease states and careful studies are needed to elucidate their therapeutic effectiveness in most indications.
Keywords: Ammonia; Cachexia; Cirrhosis; Diabetes; Glutamine; Nutrition.
Conflict of interest statement
Not applicable.The author declares that he/she has no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. - PubMed
-
- Holecek M. Leucine metabolism in fasted and tumor necrosis factor-treated rats. Clin Nutr. 1996;15:91–93. - PubMed
-
- Holecek M, Sprongl L, Skopec F, Andrýs C, Pecka M. Leucine metabolism in TNF-α- and endotoxin-treated rats: contribution of hepatic tissue Am J Phys 1997;273: E1052–E1058. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

