Cold-pressed minke whale oil reduces circulating LDL/VLDL-cholesterol, lipid oxidation and atherogenesis in apolipoprotein E-deficient mice fed a Western-type diet for 13 weeks
- PMID: 29755576
- PMCID: PMC5935995
- DOI: 10.1186/s12986-018-0269-8
Cold-pressed minke whale oil reduces circulating LDL/VLDL-cholesterol, lipid oxidation and atherogenesis in apolipoprotein E-deficient mice fed a Western-type diet for 13 weeks
Abstract
Background: Long-chain n3-polyunsaturated fatty acids (LC n3-PUFA) are well known for their anti-inflammatory activity and their impact on cardiovascular disease. Cold-pressed whale oil (CWO) has half the amount of LC n3-PUFA compared to cod liver oil (CLO). Still, there has been observed more pronounced beneficial effects on cardiovascular disease markers from intake of CWO compared to intake of CLO in human intervention studies. Extracts from CWO deprived of fatty acids have also been shown to display antioxidative and anti-inflammatory effects in vitro. The aim of this study was to investigate whether intake of a high-fat Western-type diet (WD) supplemented with CWO would prevent the development of atherosclerotic lesions in apolipoprotein E-deficient (ApoE-/-) mice.
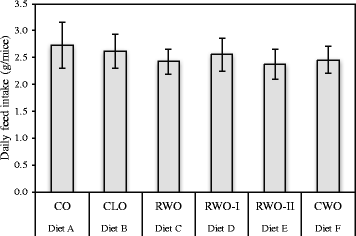
Methods: Seventy female ApoE-/- mice were fed a WD containing 1% CWO, CLO or corn oil (CO). Atherosclerotic lesion formation, body and tissue weights, hepatic gene expression together with serum levels of LDL/VLDL-cholesterol, ox-LDL, total antioxidant status and various serum cardiovascular disease/proinflammatory markers were evaluated. Statistical analyses were performed using SPSS, and Shapiro-Wilk's test was performed to determine the distribution of the variables. Statistical difference was assessed using One-Way ANOVA with Tukeys' post hoc test or Kruskal-Wallis test. The hepatic relative gene expression was analysed with REST 2009 (V2.0.13).
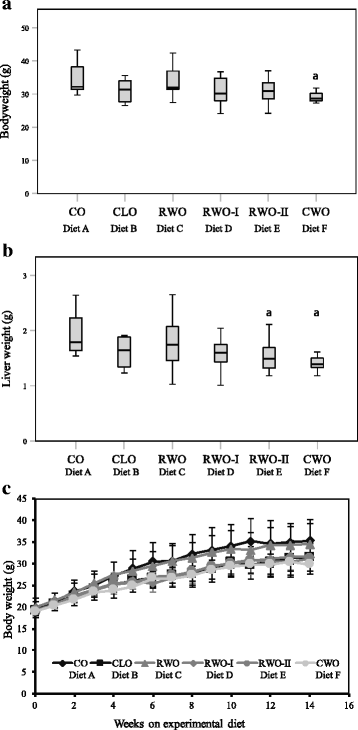
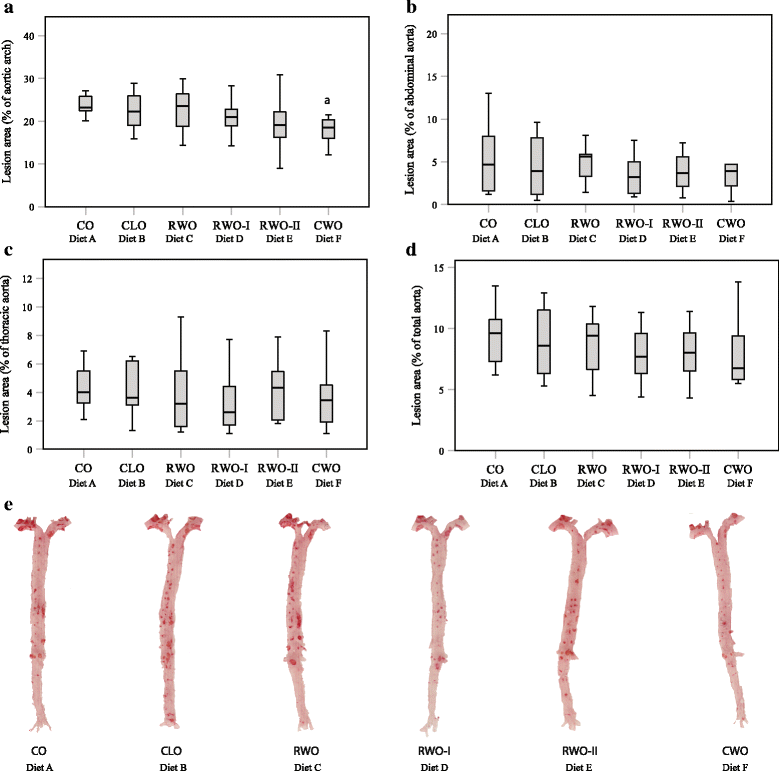
Results: Mice fed CWO had less atherosclerotic lesions in the aortic arch compared to mice fed CO. Levels of LDL/VLDL-cholesterol and ox-LDL-cholesterol were also markedly reduced whereas total antioxidant levels were enhanced in mice fed CWO compared to CO-fed mice. In addition, CWO-fed mice gained less weight and several hepatic genes involved in the cholesterol metabolism were up-regulated compared to CO-fed mice.
Conclusion: In the present study mice fed a WD supplemented with 1% CWO had reduced formation of atherosclerotic lesions in the aortic arch, reduced serum LDL/VLDL-cholesterol and ox-LDL-cholesterol, increased serum total antioxidant status and reduced body weight compared to mice fed a WD supplemented with 1% CO.
Keywords: Atherosclerosis; Balaenoptera acutorostrata; Blubber; Gene expression; LDL-cholesterol; Lesions; Plaque; VLDL-cholesterol; Whale oil.
Conflict of interest statement
The Norwegian Animal Research Authority approved the study (approval number 3828). All experiments were performed following Federation for Laboratory Animal Science Association recommendations and according to the Norwegian legislation on the care and use of experimental animals.The authors declare no competing interests, neither financial nor non-financial.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

