Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases
- PMID: 29755676
- PMCID: PMC5945519
- DOI: 10.18632/oncotarget.25041
Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases
Abstract
Brain metastases constitute a challenge in the management of patients with HER2-positive breast cancer treated with anti-HER2 systemic therapies. Here we sought to define the repertoire of mutations private to or enriched for in HER2-positive brain metastases. Massively parallel sequencing targeting all exons of 254 genes frequently mutated in breast cancers and/or related to DNA repair was used to characterize the spatial and temporal heterogeneity of HER2-positive breast cancers and their brain metastases in six patients. Data were analyzed with state-of-the-art bioinformatics algorithms and selected mutations were validated with orthogonal methods. Spatial and temporal inter-lesion genetic heterogeneity was observed in the HER2-positive brain metastases from an index patient subjected to a rapid autopsy. Genetic alterations restricted to the brain metastases included mutations in cancer genes FGFR2, PIK3CA and ATR, homozygous deletion in CDKN2A and amplification in KRAS. Shifts in clonal composition and the acquisition of additional mutations in the progression from primary HER2-positive breast cancer to brain metastases following anti-HER2 therapy were investigated in additional five patients. Likely pathogenic mutations private to or enriched in the brain lesions affected cancer and clinically actionable genes, including ATR, BRAF, FGFR2, MAP2K4, PIK3CA, RAF1 and TP53. Changes in clonal composition and the acquisition of additional mutations in brain metastases may affect potentially actionable genes in HER2-positive breast cancers. Our observations have potential clinical implications, given that treatment decisions for patients with brain metastatic disease are still mainly based on biomarkers assessed in the primary tumor.
Keywords: HER2-positive; actionable genetic alterations; brain metastasis; metastatic breast cancer; personalized medicine.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to disclose.
Figures
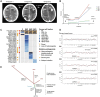

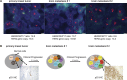
References
-
- De Ieso PB, Schick U, Rosenfelder N, Mohammed K, Ross GM. Breast cancer brain metastases - A 12 year review of treatment outcomes. Breast. 2015;24:426–33. https://doi.org/10.1016/j.breast.2015.03.007. - DOI - PubMed
-
- Gonzalez-Angulo AM, Hortobagyi GN. Brain metastases and breast cancer subtypes. Onkologie. 2010;33:143–44. https://doi.org/10.1159/000296307. - DOI - PubMed
-
- Hess KR, Esteva FJ. Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res Treat. 2013;137:449–55. https://doi.org/10.1007/s10549-012-2366-0. - DOI - PMC - PubMed
-
- Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–55. https://doi.org/10.1158/1078-0432.CCR-06-2478. - DOI - PubMed
-
- Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111:1888–98. https://doi.org/10.1038/bjc.2014.388. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

