Clinical application of targeted next-generation sequencing for colorectal cancer patients: a multicentric Belgian experience
- PMID: 29755687
- PMCID: PMC5945518
- DOI: 10.18632/oncotarget.25099
Clinical application of targeted next-generation sequencing for colorectal cancer patients: a multicentric Belgian experience
Abstract
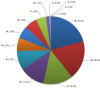
International guidelines made RAS (KRAS and NRAS) status a prerequisite for the use of anti-EGFR agents for metastatic colorectal cancer (CRC) patients. Daily, new data emerges on the theranostic and prognostic role of molecular biomarkers; this is a strong incentive for a validated, sensitive, and broadly available molecular screening test. Next-generation sequencing (NGS) has begun to supplant other technologies for genomic profiling. We report here our 2 years of clinical practice using NGS results to guide therapeutic decisions. The Ion Torrent AmpliSeq colon/lung cancer panel, which allows mutation detection in 22 cancer-related genes, was prospectively used in clinical practice (BELAC ISO 15189 accredited method). The DNA of 741 formalin-fixed paraffin-embedded CRC tissues, including primary tumors and metastasis, was obtained from 14 different Belgian institutions and subjected to targeted NGS. Of the tumors tested, 98% (727) were successfully sequenced and 89% (650) harbored at least one mutation. KRAS, BRAF and NRAS mutations were found in 335 (46%), 78 (11%) and 32 (4%) samples, respectively. These mutation frequencies were consistent with those reported in public databases. Moreover, mutations and amplifications in potentially actionable genes were identified in 464 samples (64%), including mutations in PIK3CA (14%), ERBB2 (0.4%), AKT1 (0.6%), and MAP2K1 (0.1%), as well as amplifications of ERBB2 (0.3%) and EGFR (0.3%). The median turnaround time between reception of the sample in the laboratory and report release was 8 calendar days. Overall, the AmpliSeq colon/lung cancer panel was successfully applied in daily practice and provided reliable clinically relevant information for CRC patients.
Keywords: colorectal cancer; next-generation sequencing.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared no conflicts of interest.
Figures



References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. - PubMed
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:1–9. - PubMed
-
- Shi C, Washington K. Molecular testing in colorectal cancer: diagnosis of Lynch syndrome and personalized cancer medicine. Am J Clin Pathol. 2012;137:847–859. - PubMed
-
- Allegra CJ, Rumble RB, Hamilton SR, Mangu PB, Roach N, Hantel A, Schilsky RL. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Therapy/ American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol. 2016;34:179–185. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

