A subgroup of pleural mesothelioma expresses ALK protein and may be targetable by combined rapamycin and crizotinib therapy
- PMID: 29755689
- PMCID: PMC5945506
- DOI: 10.18632/oncotarget.25111
A subgroup of pleural mesothelioma expresses ALK protein and may be targetable by combined rapamycin and crizotinib therapy
Abstract
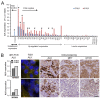
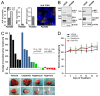
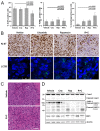
Malignant pleural mesothelioma (MPM) is a neoplasm with inferior prognosis and notorious chemotherapeutic resistance. Targeting aberrantly overexpressed kinases to cure MPM is a promising therapeutic strategy. Here, we examined ALK, MET and mTOR as potential therapeutic targets and determined the combinatorial efficacy of ALK and mTOR targeting on tumor cell growth in vivo. First, ALK overexpression, rearrangement and mutation were studied in primary MPM by qRT-PCR, FISH, immunohistochemistry and sequence analysis; mTOR and MET expression by qRT-PCR and immunohistochemistry. Overexpression of full-length ALK transcripts was observed in 25 (19.5%) of 128 primary MPM, of which ten expressed ALK protein. ALK overexpression was not associated with gene rearrangement, amplification or kinase-domain mutation. mTOR protein was detected in 28.7% MPM, co-expressed with ALK or MET in 5% and 15% MPM, respectively. The ALK/MET inhibitor crizotinib enhanced the anti-tumor effect of the mTOR-inhibitor rapamycin in a patient-derived MPM xenograft with co-activated ALK/mTOR: combined therapy achieved tumor shrinkage in 4/5 tumors and growth stagnation in one tumor. Treatment effects on proliferation, apoptosis, autophagy and pathway signaling were assessed using Ki-67 immunohistochemistry, TUNEL assay, LC3B immunofluorescence, and immunoblotting. Co-treatment significantly suppressed cell proliferation and induced autophagy and caspase-independent, necrotic cell death. Rapamycin/crizotinib simultaneously inhibited mTORC1 (evidenced by S6 kinase and RPS6 dephosphorylation) and ALK signaling (ALK, AKT, STAT3 dephosphorylation), and crizotinib suppressed the adverse AKT activation induced by rapamycin. In conclusion, co-treatment with rapamycin and crizotinib is effective in suppressing MPM tumor growth and should be further explored as a therapeutic alternative in mesothelioma.
Keywords: ALK; combination therapy; crizotinib; pleural mesothelioma; rapamycin.
Conflict of interest statement
CONFLICTS OF INTEREST All authors have no conflicts of interest to declare.
Figures

References
-
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. - PubMed
-
- Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin C, Hillerdal G, Le Péchoux C, Mutti L, Pairon JC, et al. Guidelines of the european respiratory society and the european society of thoracic surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479–495. - PubMed
-
- Roengvoraphoj M, Tsongalis GJ, Dragnev KH, Rigas JR. Epidermal growth factor receptor tyrosine kinase inhibitors as initial therapy for non-small cell lung cancer: focus on epidermal growth factor receptor mutation testing and mutation-positive patients. Cancer Treat Rev. 2013;39:839–850. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

