Decolonization of Intestinal Carriage of MDR/XDR Gram-Negative Bacteria with Oral Colistin in Patients with Hematological Malignancies: Results of a Randomized Controlled Trial
- PMID: 29755707
- PMCID: PMC5937973
- DOI: 10.4084/MJHID.2018.030
Decolonization of Intestinal Carriage of MDR/XDR Gram-Negative Bacteria with Oral Colistin in Patients with Hematological Malignancies: Results of a Randomized Controlled Trial
Abstract
Background: Intestinal colonization by MDR/XDR gram-negative bacteria leads to an increased risk of subsequent bloodstream infections (BSI) in patients receiving chemotherapy as a treatment for hematologic malignancies.
Objectives: The objective of this study was to evaluate the efficacy of oral colistin in eradicating the intestinal carriage of MDR/XDR Gram-negative bacteria in patients with hematological malignancies.
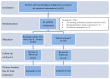
Methods: In a tertiary hematology center, adult patients with intestinal colonization by MDR/XDR Gram-negative bacteria were included in a randomized controlled trial (RCT) during a period from November 2016 to October 2017. Patients were treated with oral colistin for 14 days or observed with the primary outcome set as decolonization on day 21 post-treatment. Secondary outcomes included treatment safety and changes in MICs of isolated microorganisms. ClinicalTrials.gov Identifier: NCT02966457.
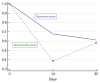
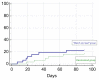
Results: Short-time positive effect (61.3% vs 32.3%; OR 3.32; 95% CI 1.17-9.44; p=0.0241) was demonstrated on the day 14 of colistin treatment, without any statistical difference on day 21 post-treatment. The incidence of BSI in decolonization group was lower in the first 30 days after the intervention (3.2% vs. 12.9%), but overall in the 90-day observation period, it did not show any advantages comparing to control group (log-rank test; p=0.4721). No serious adverse effects or increase in resistance to colistin was observed.
Conclusions: This study suggests that in hematological patients the strategy of selective intestinal decolonization by colistin may be beneficial to decrease the rate of MDR/XDR Gram-negative intestinal colonization and the risk of BSI in the short-term period, having no long-term sustainable effects.
Keywords: Hematology; Multidrug-resistant bacteria; Neutropenia; Polymyxins; Selective oral decolonization.
Conflict of interest statement
Competing interests: The authors have declared that no competing interests exist.
Figures
References
-
- Birgand G, Armand-Lefevre L, Lolom I, Ruppe E, Andremont A, Lucet JC. Duration of colonization by extended-spectrum β-lactamase-producing Enterobacteriaceaeafter hospital discharge. Am J Infect Control. 2013;41(5):443–7. https://doi.org/10.1016/j.ajic.2012.05.015. - DOI - PubMed
-
- Löhr IH, Rettedal S, Natås OB, Naseer U, Oymar K, Sundsfjord A. Long-termfaecal carriage in infants and intra-household transmission of CTX-M-15-producingKlebsiella pneumoniae following a nosocomial outbreak. J Antimicrob Chemother. 2013 May;68(5):1043–8. https://doi.org/10.1093/jac/dks502. - DOI - PubMed
-
- Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008;300(24):2911–3. https://doi.org/10.1001/jama.2008.896. - DOI - PubMed
-
- Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ International Klebseilla Study Group. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13(7):986–93. https://doi.org/10.3201/eid1307.070187. - DOI - PMC - PubMed
-
- Denis B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E, Hennequin C, Allez M, Socie G, Maziers N, Porcher R, Molina JM. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia: a five-year study. Int J Infect Dis. 2015;39:1–6. https://doi.org/10.1016/j.ijid.2015.07.010. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

