Sofosbuvir Plus Daclatasvir in Treatment of Chronic Hepatitis C Genotype 4 Infection in a Cohort of Egyptian Patients: An Experiment the Size of Egyptian Village
- PMID: 29755792
- PMCID: PMC5884208
- DOI: 10.1155/2018/9616234
Sofosbuvir Plus Daclatasvir in Treatment of Chronic Hepatitis C Genotype 4 Infection in a Cohort of Egyptian Patients: An Experiment the Size of Egyptian Village
Abstract
Background and aims: As indicated by the World Health Organization (WHO), Egypt is positioned as the country with the world's highest prevalence of Hepatitis C virus (HCV). HCV is transmitted through unexamined blood transfusions, different employments of syringes, and poor cleansing, as per the WHO. Our study aimed at screening and management of chronic hepatitis C genotype 4 infected patients in Bardeen village, Sharkeya Governorate, Egypt, with Sofosbuvir plus Daclatasvir, as well as estimating the safety and efficacy of that regimen.
Methods: Screening of adult patients in Bardeen village was done from March 2016 till November 2016 using hepatitis C virus antibodies by third-generation ELISA testing. Positive results were confirmed by PCR. Patients eligible for treatment received Sofosbuvir 400 mg and Daclatasvir 60 mg daily for 12 weeks and were assessed for sustained virologic response at 12 weeks following the end of treatment (SVR 12).
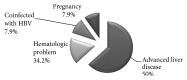
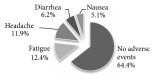
Results: Out of 2047 subjects screened for hepatitis C virus, 249 (12.2%) showed positive results. 221 out of those 249 subjects (88.7%) had detectable RNA by PCR. Treatment of eligible patients (183 patients) with Sofosbuvir plus Daclatasvir for 12 weeks resulted in 96% achievement of sustained virologic response at week 12. Adverse events were tolerable.
Conclusion: Sofosbuvir plus Daclatasvir regimen is safe and effective for treatment of chronic hepatitis C Genotype 4 infected patients with minimal adverse events. HCV eradication program implemented in Egypt can be a model for other countries with HCV and limited resources. The availability of generic drugs in Egypt will help much in eradication of the virus.
Figures
References
-
- Aly Abd Elrazek A. E. M., Bilasy S. E., Elbanna A. E. M., Elsherif A. E. A. Prior to the oral therapy, what do we know about HCV-4 in Egypt: a randomized survey of prevalence and risks using data mining computed analysis. Medicine (United States) 2014;93(28):p. e204. doi: 10.1097/MD.0000000000000204. - DOI - PMC - PubMed
-
- Elwan N., Elfert A., Abd-Elsalam S., et al. Study of hepatic steatosis index in patients with chronic HCV infection. International Journal of Current Microbiology and Applied Sciences. 2016;5(5):266–279. doi: 10.20546/ijcmas.2016.505.029. - DOI
-
- Abd-Elsalam S., Sharaf-Eldin M., Soliman S., Elfert A., Badawi R., Ahmad Y. K. Efficacy and safety of sofosbuvir plus ribavirin for treatment of cirrhotic patients with genotype 4 hepatitis C virus in real-life clinical practice. Archives of Virology. 2017:1–6. doi: 10.1007/s00705-017-3573-0. - DOI - PubMed
-
- Ahmed O. A., Kaisar H. H., Hawash N., et al. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in treatment of a cohort of egyptian patients with hepatitis C virus infection. Infectious Disorders—Drug Targets. 2017;17(2):95–100. doi: 10.2174/1871526517666170417143216. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials