Cholinergic excitation complements glutamate in coding visual information in retinal ganglion cells
- PMID: 29758086
- PMCID: PMC6092279
- DOI: 10.1113/JP275073
Cholinergic excitation complements glutamate in coding visual information in retinal ganglion cells
Abstract
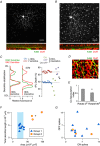
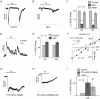
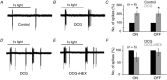
Starburst amacrine cells release GABA and ACh. This study explores the coordinated function of starburst-mediated cholinergic excitation and GABAergic inhibition to bistratified retinal ganglion cells, predominantly direction-selective ganglion cells (DSGCs). In rat retina, under our recording conditions, starbursts were found to provide the major excitatory drive to a sub-population of ganglion cells whose dendrites co-stratify with starburst dendrites (putative DSGCs). In mouse retina, recordings from genetically identified DSGCs at physiological temperatures reveal that ACh inputs dominate the response to small spot-high contrast light stimuli, with preferential addition of bipolar cell input shifting the balance towards glutamate for larger spot stimuli In addition, starbursts also appear to gate glutamatergic excitation to DSGCs by postsynaptic and possibly presynaptic inhibitory processes ABSTRACT: Starburst amacrine cells release both GABA and ACh, allowing them to simultaneously mediate inhibition and excitation. However, the precise pre- and postsynaptic targets for ACh and GABA remain under intense investigation. Most previous studies have focused on starburst-mediated postsynaptic GABAergic inhibition and its role in the formation of directional selectivity in ganglion cells. However, the significance of postsynaptic cholinergic excitation is only beginning to be appreciated. Here, we found that light-evoked responses measured in bi-stratified rat ganglion cells with dendrites that co-fasciculate with ON and OFF starburst dendrites (putative direction-selective ganglion cells, DSGCs) were abolished by the application of nicotinic receptor antagonists, suggesting ACh could act as the primary source of excitation. Recording from genetically labelled DSGCs in mouse retina at physiological temperatures revealed that cholinergic synaptic inputs dominated the excitation for high contrast stimuli only when the size of the stimulus was small. Canonical glutamatergic inputs mediated by bipolar cells were prominent when GABA/glycine receptors were blocked or when larger spot stimuli were utilized. In mouse DSGCs, bipolar cell excitation could also be unmasked through the activation of mGluR2,3 receptors, which we show suppresses starburst output, suggesting that GABA from starbursts serves to inhibit bipolar cell signals in DSGCs. Taken together, these results suggest that starbursts amplify excitatory signals traversing the retina, endowing DSGCs with the ability to encode fine spatial information without compromising their ability to encode direction.
Keywords: acetylcholine; feedback inhibition; starburst amacrine cells.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures



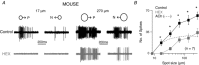
Similar articles
-
Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning.J Neurosci. 2014 Mar 12;34(11):3976-81. doi: 10.1523/JNEUROSCI.5017-13.2014. J Neurosci. 2014. PMID: 24623775 Free PMC article.
-
Conditional Knock-Out of Vesicular GABA Transporter Gene from Starburst Amacrine Cells Reveals the Contributions of Multiple Synaptic Mechanisms Underlying Direction Selectivity in the Retina.J Neurosci. 2015 Sep 23;35(38):13219-32. doi: 10.1523/JNEUROSCI.0933-15.2015. J Neurosci. 2015. PMID: 26400950 Free PMC article.
-
A Central Role for Mixed Acetylcholine/GABA Transmission in Direction Coding in the Retina.Neuron. 2016 Jun 15;90(6):1243-1256. doi: 10.1016/j.neuron.2016.04.041. Epub 2016 May 26. Neuron. 2016. PMID: 27238865
-
Biophysical mechanisms and essential topography of directionally selective subunits in rabbit's retina.J Integr Neurosci. 2005 Sep;4(3):341-61. doi: 10.1142/s0219635205000860. J Integr Neurosci. 2005. PMID: 16178062 Review.
-
On and off pathways through amacrine cells in mammalian retina: the synaptic connections of "starburst" amacrine cells.Vision Res. 1983;23(11):1265-79. doi: 10.1016/0042-6989(83)90102-5. Vision Res. 1983. PMID: 6362185 Review.
Cited by
-
Realistic retinal modeling unravels the differential role of excitation and inhibition to starburst amacrine cells in direction selectivity.PLoS Comput Biol. 2021 Dec 30;17(12):e1009754. doi: 10.1371/journal.pcbi.1009754. eCollection 2021 Dec. PLoS Comput Biol. 2021. PMID: 34968385 Free PMC article.
-
Neural mechanisms of contextual modulation in the retinal direction selective circuit.Nat Commun. 2019 Jun 3;10(1):2431. doi: 10.1038/s41467-019-10268-z. Nat Commun. 2019. PMID: 31160566 Free PMC article.
-
Neural Circuits Underlying Multifeature Extraction in the Retina.J Neurosci. 2024 Mar 6;44(10):e0910232023. doi: 10.1523/JNEUROSCI.0910-23.2023. J Neurosci. 2024. PMID: 37957014 Free PMC article.
-
ON and OFF starburst amacrine cells are controlled by distinct cholinergic pathways.J Gen Physiol. 2024 Aug 5;156(8):e202413550. doi: 10.1085/jgp.202413550. Epub 2024 Jun 5. J Gen Physiol. 2024. PMID: 38836782 Free PMC article.
-
Activity-dependent development of synaptic circuits mediates direction selectivity in an axis-specific manner.Cell Rep. 2025 Jul 22;44(7):115897. doi: 10.1016/j.celrep.2025.115897. Epub 2025 Jun 24. Cell Rep. 2025. PMID: 40560726 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

