Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond
- PMID: 29758235
- PMCID: PMC7126013
- DOI: 10.1016/j.antiviral.2018.05.005
Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond
Abstract
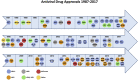
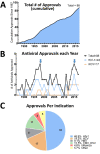
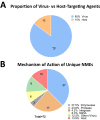
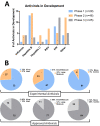
2017 marked the 30th anniversary of the approval of zidovudine (AZT) as the first HIV/AIDS therapy. Since then, more than eighty antiviral drugs have received FDA approval, half of which treat HIV infection. Here, we provide a retrospective analysis of approved antiviral drugs, including therapeutics against other major chronic infections such as hepatitis B and C, and herpes viruses, over the last thirty years. During this time, only a few drugs were approved to treat acute viral infections, mainly influenza. Analysis of these approved antiviral drugs based on molecular class and mode of action shows that a large majority are small molecules and direct-acting agents as opposed to proteins, peptides, or oligonucleotides and host-targeting therapies. In addition, approvals of combination therapies accelerated over the last five years. We also provide a prospective study of future potential antiviral therapies, based on current clinical research pipelines across the pharmaceutical industry. Comparing past drug approvals with current clinical candidates hints at the future evolution in antiviral therapies and reveals how antiviral medicines are often discovered. Overall, this work helps forecast future trends and innovation in the field of antiviral research and development.
Keywords: Antiviral; Chronic viral infection; Direct acting agent; Respiratory virus; Small molecule.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Bagga B., Woods C.W., Veldman T.H., Gilbert A., Mann A., Balaratnam G., Lambkin-Williams R., Oxford J.S., McClain M.T., Wilkinson T., Nicholson B.P., Ginsburg G.S., Devincenzo J.P. Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir. Ther. 2013;18:785–791. - PubMed
-
- Belema M., Meanwell N.A. Discovery of daclatasvir, a pan-genotypic hepatitis C virus NS5A replication complex inhibitor with potent clinical effect. J. Med. Chem. 2014;57:5057–5071. - PubMed
-
- Burke B.P., Levin B.R., Zhang J., Sahakyan A., Boyer J., Carroll M.V., Colon J.C., Keech N., Rezek V., Bristol G., Eggers E., Cortado R., Boyd M.P., Impey H., Shimizu S., Lowe E.L., Ringpis G.E., Kim S.G., Vatakis D.N., Breton L.R., Bartlett J.S., Chen I.S., Kitchen S.G., An D.S., Symonds G.P. Engineering cellular resistance to HIV-1 infection in vivo using a dual therapeutic lentiviral vector. Molecular therapy. Nucleic Acids. 2015;4:e236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

