Inhibition of 2-AG hydrolysis differentially regulates blood brain barrier permeability after injury
- PMID: 29759062
- PMCID: PMC5952841
- DOI: 10.1186/s12974-018-1166-9
Inhibition of 2-AG hydrolysis differentially regulates blood brain barrier permeability after injury
Abstract
Background: Acute neurological insults caused by infection, systemic inflammation, ischemia, or traumatic injury are often associated with breakdown of the blood-brain barrier (BBB) followed by infiltration of peripheral immune cells, cytotoxic proteins, and water. BBB breakdown and extravasation of these peripheral components into the brain parenchyma result in inflammation, oxidative stress, edema, excitotoxicity, and neurodegeneration. These downstream consequences of BBB dysfunction can drive pathophysiological processes and play a substantial role in the morbidity and mortality of acute and chronic neurological insults, and contribute to long-term sequelae. Preserving or rescuing BBB integrity and homeostasis therefore represents a translational research area of high therapeutic potential.
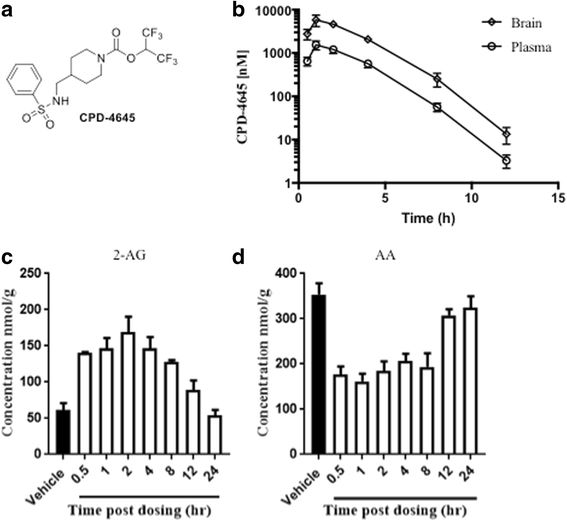
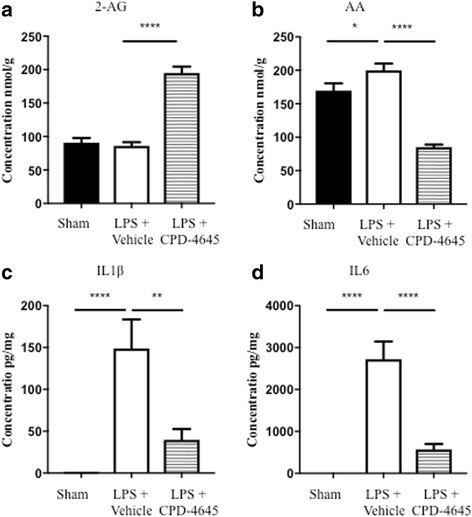
Methods: Induction of general and localized BBB disruption in mice was carried out using systemic administration of LPS and focal photothrombotic ischemic insult, respectively, in the presence and absence of the monoacylglycerol lipase (MAGL) inhibitor, CPD-4645. The effects of CPD-4645 treatment were assessed by gene expression analysis performed on neurovascular-enriched brain fractions, cytokine and inflammatory mediator measurement, and functional assessment of BBB permeability. The mechanism of action of CPD-4645 was studied pharmacologically using inverse agonists/antagonists of the cannabinoid receptors CB1 and CB2.
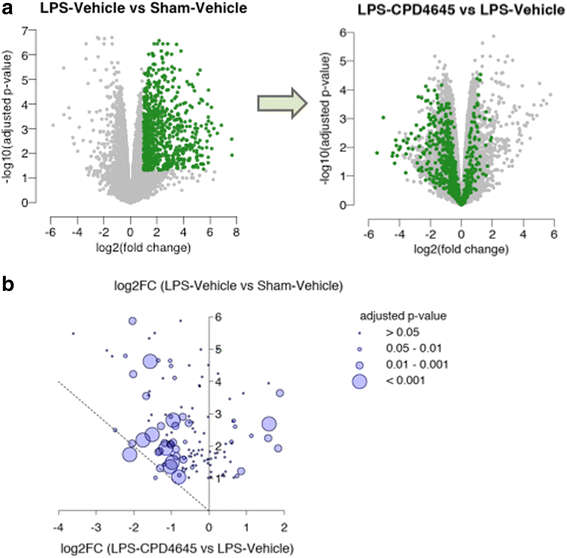
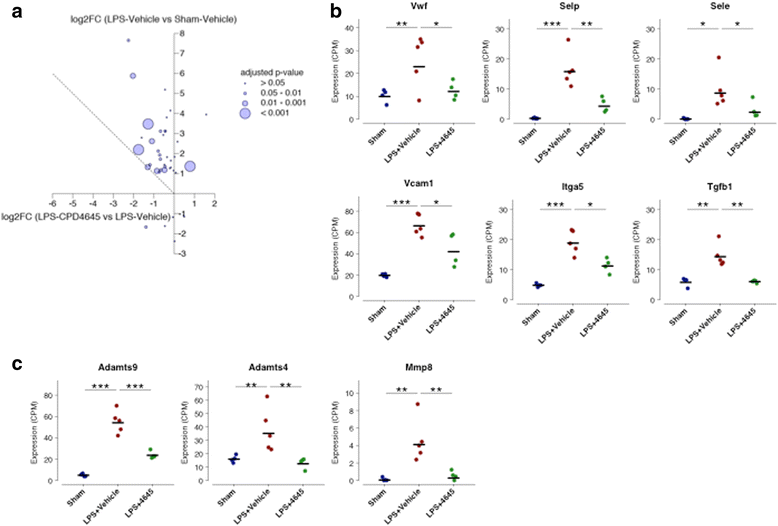
Results: Here, we demonstrate that the neurovasculature exhibits a unique transcriptional signature following inflammatory insults, and pharmacological inhibition of MAGL using a newly characterized inhibitor rescues the transcriptional profile of brain vasculature and restores its functional homeostasis. This pronounced effect of MAGL inhibition on blood-brain barrier permeability is evident following both systemic inflammatory and localized ischemic insults. Mechanistically, the protective effects of the MAGL inhibitor are partially mediated by cannabinoid receptor signaling in the ischemic brain insult.
Conclusions: Our results support considering MAGL inhibitors as potential therapeutics for BBB dysfunction and cerebral edema associated with inflammatory brain insults.
Keywords: 2-arachidonoylglycerol; Blood-brain barrier; Monoacylglycerol lipase; Neuroinflammation; Neurovasculature.
Conflict of interest statement
Ethics approval
All procedures involving animals were conducted with the approval of the Pfizer Institute Animal Care and Use Committee (IACUC) and compliant with the regulations and standards of the Animal Welfare Act.
Competing interests
JRP, GLS, JQ, YP, SMO, MI, NP, TAL, HX, RDB, and TAS are or were current employees of Pfizer Inc. at the time the studies were conducted.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

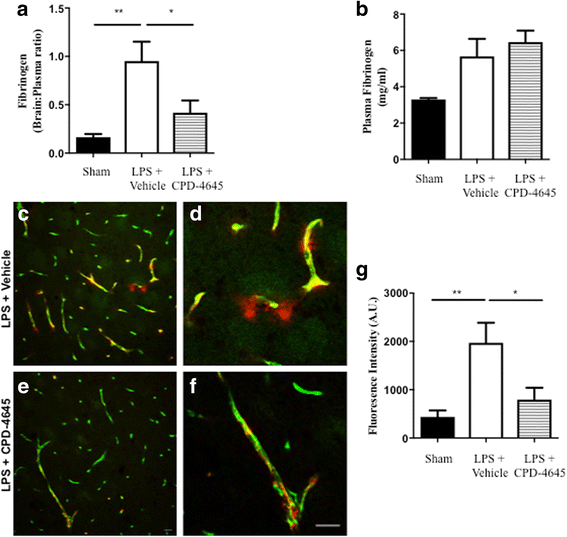
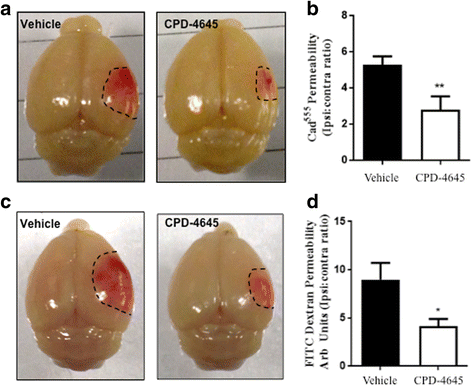

References
-
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun. 2009;23(4):507–517. doi: 10.1016/j.bbi.2009.01.017. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

