Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells
- PMID: 29759145
- PMCID: PMC5967628
- DOI: 10.1016/j.jneuroim.2018.04.018
Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells
Abstract
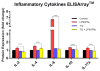
Neuroinflammation and microglial activation are pathological markers of a number of central nervous system (CNS) diseases. Chronic activation of microglia induces the release of excessive amounts of reactive oxygen species (ROS) and pro-inflammatory cytokines. Additionally, chronic microglial activation has been implicated in several neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease. Thymoquinone (TQ) has been identified as one of the major active components of the natural product Nigella sativa seed oil. TQ has been shown to exhibit anti-inflammatory, anti-oxidative, and neuroprotective effects. In this study, lipopolysaccharide (LPS) and interferon gamma (IFNγ) activated BV-2 microglial cells were treated with TQ (12.5 μM for 24 h). We performed quantitative proteomic analysis using Orbitrap/Q-Exactive Proteomic LC-MS/MS (Liquid chromatography-mass spectrometry) to globally assess changes in protein expression between the treatment groups. Furthermore, we evaluated the ability of TQ to suppress the inflammatory response using ELISArray™ for Inflammatory Cytokines. We also assessed TQ's effect on the gene expression of NFκB signaling targets by profiling 84 key genes via real-time reverse transcription (RT2) PCR array. Our results indicated that TQ treatment of LPS/IFNγ-activated microglial cells significantly increased the expression of 4 antioxidant, neuroprotective proteins: glutaredoxin-3 (21 fold; p < 0.001), biliverdin reductase A (15 fold; p < 0.0001), 3-mercaptopyruvate sulfurtransferase (11 fold; p < 0.01), and mitochondrial lon protease (>8 fold; p < 0.001) compared to the untreated, activated cells. Furthermore, TQ treatment significantly (P < 0.0001) reduced the expression of inflammatory cytokines, IL-2 = 38%, IL-4 = 19%, IL-6 = 83%, IL-10 = 237%, and IL-17a = 29%, in the activated microglia compared to the untreated, activated which expression levels were significantly elevated compared to the control microglia: IL-2 = 127%, IL-4 = 151%, IL-6 = 670%, IL-10 = 133%, IL-17a = 127%. Upon assessing the gene expression of NFκB signaling targets, this study also demonstrated that TQ treatment of activated microglia resulted in >7 fold down-regulation of several NFκB signaling targets genes, including interleukin 6 (IL6), complement factor B (CFB), chemokine (CC motif) ligand 3 (CXCL3), chemokine (CC) motif ligand 5 (CCL5) compared to the untreated, activated microglia. This modulation in gene expression counteracts the >10-fold upregulation of these same genes observed in the activated microglia compared to the controls. Our results show that TQ treatment of LPS/IFNγ-activated BV-2 microglial cells induce a significant increase in expression of neuroprotective proteins, a significant decrease in expression inflammatory cytokines, and a decrease in the expression of signaling target genes of the NFκB pathway. Our findings are the first to show that TQ treatment increased the expression of these neuroprotective proteins (biliverdin reductase-A, 3-mercaptopyruvate sulfurtransferase, glutaredoxin-3, and mitochondrial lon protease) in the activated BV-2 microglial cells. Additionally, our results indicate that TQ treatment decreased the activation of the NFκB signaling pathway, which plays a key role in neuroinflammation. In conclusion, our results demonstrate that TQ treatment reduces the inflammatory response and modulates the expression of specific proteins and genes and hence potentially reduce neuroinflammation and neurodegeneration driven by microglial activation.
Keywords: Microglia; NFκB; Neuroinflammation; Neuroprotection; Thymoquinone.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
None declared.
Figures
Similar articles
-
The Antioxidant Effects of Thymoquinone in Activated BV-2 Murine Microglial Cells.Neurochem Res. 2016 Dec;41(12):3227-3238. doi: 10.1007/s11064-016-2047-1. Epub 2016 Sep 1. Neurochem Res. 2016. PMID: 27585756 Free PMC article.
-
Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells.J Neuroimmunol. 2015 Sep 15;286:5-12. doi: 10.1016/j.jneuroim.2015.06.011. Epub 2015 Jun 27. J Neuroimmunol. 2015. PMID: 26298318 Free PMC article.
-
LXW7 attenuates inflammation via suppressing Akt/nuclear factor kappa B and mitogen-activated protein kinases signaling pathways in lipopolysaccharide-stimulated BV2 microglial cells.Int Immunopharmacol. 2019 Dec;77:105963. doi: 10.1016/j.intimp.2019.105963. Epub 2019 Nov 13. Int Immunopharmacol. 2019. PMID: 31732449
-
Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19.Pharm Biol. 2021 Dec;59(1):696-703. doi: 10.1080/13880209.2021.1931353. Pharm Biol. 2021. PMID: 34110959 Free PMC article. Review.
-
Therapeutic potential of plant-derived natural compounds in Alzheimer's disease: Targeting microglia-mediated neuroinflammation.Biomed Pharmacother. 2024 Sep;178:117235. doi: 10.1016/j.biopha.2024.117235. Epub 2024 Aug 1. Biomed Pharmacother. 2024. PMID: 39094545 Review.
Cited by
-
Alzheimer's disease: natural products as inhibitors of neuroinflammation.Inflammopharmacology. 2020 Dec;28(6):1439-1455. doi: 10.1007/s10787-020-00751-1. Epub 2020 Sep 15. Inflammopharmacology. 2020. PMID: 32930914 Free PMC article. Review.
-
Differentiation and regulation of CD4+ T cell subsets in Parkinson's disease.Cell Mol Life Sci. 2024 Aug 17;81(1):352. doi: 10.1007/s00018-024-05402-0. Cell Mol Life Sci. 2024. PMID: 39153043 Free PMC article. Review.
-
SOCS1/JAK2/STAT3 axis regulates early brain injury induced by subarachnoid hemorrhage via inflammatory responses.Neural Regen Res. 2021 Dec;16(12):2453-2464. doi: 10.4103/1673-5374.313049. Neural Regen Res. 2021. PMID: 33907034 Free PMC article.
-
Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure.Drug Des Devel Ther. 2021 May 3;15:1819-1833. doi: 10.2147/DDDT.S308863. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33976534 Free PMC article. Review.
-
Thymoquinone in Ocular Neurodegeneration: Modulation of Pathological Mechanisms via Multiple Pathways.Front Cell Neurosci. 2022 Mar 2;16:786926. doi: 10.3389/fncel.2022.786926. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35308121 Free PMC article. Review.
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mark R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383–421. - PMC - PubMed
-
- Alhebshi AH, Gotoh M, Suzuki I. Thymoquinone protects cultured rat primary neurons against amyloid β-induced neurotoxicity. Biochem Biophys Res Commun. 2013;433(4):362–367. - PubMed
-
- Alhebshi AH, Odawara A, Gotoh M, Suzuki I. Thymoquinone protects cultured hippocampal and human induced pluripotent stem cell-derived neurons against α-synuclein-induced synapse damage. Neurosci Lett. 2014;570:126–131. - PubMed
-
- Al-Majed AA, Al-Omar FA, Nagi MN. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol. 2006;543(1–3):40–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

