A Mobile Health Intervention to Reduce Pain and Improve Health (MORPH) in Older Adults With Obesity: Protocol for the MORPH Trial
- PMID: 29759957
- PMCID: PMC5972205
- DOI: 10.2196/resprot.9712
A Mobile Health Intervention to Reduce Pain and Improve Health (MORPH) in Older Adults With Obesity: Protocol for the MORPH Trial
Abstract
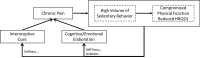
Background: Chronic pain is a complex, age-related health issue that affects both physical functioning and quality of life. Because the impact of chronic pain is worsened by obesity and inactivity, nonpharmacological interventions that promote movement, reduce sitting, and aid in weight loss are needed to help manage pain symptoms among older adults with chronic pain.
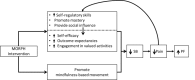
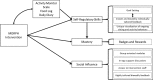
Objective: The Mobile Intervention to Reduce Pain and Improve Health (MORPH) pilot trial aims to develop and test the feasibility and acceptability of a novel, patient-centered intervention to reduce chronic pain and improve physical functioning in older adults, leveraging the combination of telecoaching and individually adaptive mHealth tools to decrease both body mass and sedentary behavior.
Methods: MORPH comprises 2 phases, including a 1-year iterative development phase, and a 1-year pilot randomized controlled trial (RCT). During the development phase, representative participants will engage in one-on-one structured interviews and a 1-week field test. The resulting feedback will be used to guide the development of the finalized MORPH intervention package. During the second phase, the finalized intervention will be tested in a pilot RCT (N=30) in which older adult participants with chronic pain and obesity will be assigned to receive the 12-week MORPH intervention or to a waitlist control. Primary outcomes include self-reported pain symptoms and physical function.
Results: Phase 1 recruitment is ongoing as of December 2017.
Conclusions: The MORPH intervention brings together a strong body of evidence using group-based behavioral intervention designs with contemporary mHealth principles, allowing for intervention when and where it matters the most. Given the ubiquity of smartphone devices and the popularity of consumer activity and weight monitors, the results of this study may serve to inform the development of scalable, socially driven behavioral pain management interventions.
Trial registration: ClinicalTrials.gov NCT03377634; https://clinicaltrials.gov/ct2/show/NCT03377634 (Archived by WebCite at http://www.webcitation.org/6yj0J5Pan).
Registered report identifier: RR1-10.2196/9712.
Keywords: chronic pain; mindfulness; sedentary lifestyle; technology; weight loss.
©Jason Fanning, Amber K Brooks, Edward Ip, Barbara J Nicklas, W Jack Rejeski. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 14.05.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


Similar articles
-
A Mobile Health Behavior Intervention to Reduce Pain and Improve Health in Older Adults With Obesity and Chronic Pain: The MORPH Pilot Trial.Front Digit Health. 2020 Dec;2:598456. doi: 10.3389/fdgth.2020.598456. Epub 2020 Dec 18. Front Digit Health. 2020. PMID: 33817686 Free PMC article.
-
Building on Lessons Learned in a Mobile Intervention to Reduce Pain and Improve Health (MORPH): Protocol for the MORPH-II Trial.JMIR Res Protoc. 2021 Jul 19;10(7):e29013. doi: 10.2196/29013. JMIR Res Protoc. 2021. PMID: 34279241 Free PMC article.
-
Successes and lessons learned from a mobile health behavior intervention to reduce pain and improve health in older adults with obesity and chronic pain: a qualitative study.Front Pain Res (Lausanne). 2024 Apr 25;5:1340400. doi: 10.3389/fpain.2024.1340400. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 38726351 Free PMC article.
-
A Self-Directed Mobile Intervention (WaznApp) to Promote Weight Control Among Employees at a Lebanese University: Protocol for a Feasibility Pilot Randomized Controlled Trial.JMIR Res Protoc. 2018 May 16;7(5):e133. doi: 10.2196/resprot.9793. JMIR Res Protoc. 2018. PMID: 29769174 Free PMC article.
-
Reducing Sedentary Time for Obese Older Adults: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2018 Feb 12;7(2):e23. doi: 10.2196/resprot.8883. JMIR Res Protoc. 2018. PMID: 29434012 Free PMC article.
Cited by
-
Developing optimized physical activity interventions for drug-resistant epilepsy: Challenges and lessons learned from a remote exercise intervention pilot trial.Epilepsy Behav Rep. 2024 Jun 29;27:100693. doi: 10.1016/j.ebr.2024.100693. eCollection 2024. Epilepsy Behav Rep. 2024. PMID: 39416712 Free PMC article.
-
A Mobile Health Behavior Intervention to Reduce Pain and Improve Health in Older Adults With Obesity and Chronic Pain: The MORPH Pilot Trial.Front Digit Health. 2020 Dec;2:598456. doi: 10.3389/fdgth.2020.598456. Epub 2020 Dec 18. Front Digit Health. 2020. PMID: 33817686 Free PMC article.
-
A Pain eHealth Platform for Engaging Obese, Older Adults with Chronic Low Back Pain in Nonpharmacological Pain Treatments: Protocol for a Pilot Feasibility Study.JMIR Res Protoc. 2020 Jan 2;9(1):e14525. doi: 10.2196/14525. JMIR Res Protoc. 2020. PMID: 31895042 Free PMC article.
-
Building on Lessons Learned in a Mobile Intervention to Reduce Pain and Improve Health (MORPH): Protocol for the MORPH-II Trial.JMIR Res Protoc. 2021 Jul 19;10(7):e29013. doi: 10.2196/29013. JMIR Res Protoc. 2021. PMID: 34279241 Free PMC article.
-
Perspectives and Experiences on eHealth Solutions for Coping With Chronic Pain: Qualitative Study Among Older People Living With Chronic Pain.JMIR Aging. 2024 Sep 5;7:e57196. doi: 10.2196/57196. JMIR Aging. 2024. PMID: 39235831 Free PMC article.
References
-
- Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21(6):513–23.00002508-200511000-00008 - PubMed
-
- Eggermont LH, Leveille SG, Shi L, Kiely DK, Shmerling RH, Jones RN, Guralnik JM, Bean JF. Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc. 2014 Jun;62(6):1007–16. doi: 10.1111/jgs.12848. http://europepmc.org/abstract/MED/24823985 - DOI - PMC - PubMed
-
- McCarberg BH, Nicholson BD, Todd KH, Palmer T, Penles L. The impact of pain on quality of life and the unmet needs of pain management: results from pain sufferers and physicians participating in an Internet survey. Am J Ther. 2008;15(4):312–20. doi: 10.1097/MJT.0b013e31818164f2.00045391-200807000-00004 - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

