Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing
- PMID: 29760099
- PMCID: PMC5984524
- DOI: 10.1073/pnas.1801013115
Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing
Abstract
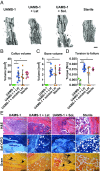
Orthopedic implant infections are a significant clinical problem, with current therapies limited to surgical debridement and systemic antibiotic regimens. Lysostaphin is a bacteriolytic enzyme with high antistaphylococcal activity. We engineered a lysostaphin-delivering injectable PEG hydrogel to treat Staphylococcus aureus infections in bone fractures. The injectable hydrogel formulation adheres to exposed tissue and fracture surfaces, ensuring efficient, local delivery of lysostaphin. Lysostaphin encapsulation within this synthetic hydrogel maintained enzyme stability and activity. Lysostaphin-delivering hydrogels exhibited enhanced antibiofilm activity compared with soluble lysostaphin. Lysostaphin-delivering hydrogels eradicated S. aureus infection and outperformed prophylactic antibiotic and soluble lysostaphin therapy in a murine model of femur fracture. Analysis of the local inflammatory response to infections treated with lysostaphin-delivering hydrogels revealed indistinguishable differences in cytokine secretion profiles compared with uninfected fractures, demonstrating clearance of bacteria and associated inflammation. Importantly, infected fractures treated with lysostaphin-delivering hydrogels fully healed by 5 wk with bone formation and mechanical properties equivalent to those of uninfected fractures, whereas fractures treated without the hydrogel carrier were equivalent to untreated infections. Finally, lysostaphin-delivering hydrogels eliminate methicillin-resistant S. aureus infections, supporting this therapy as an alternative to antibiotics. These results indicate that lysostaphin-delivering hydrogels effectively eliminate orthopedic S. aureus infections while simultaneously supporting fracture repair.
Keywords: S. aureus; biomaterials; infection; lysostaphin; orthopedics.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: C.T.J. and A.J.G. are inventors on a patent application filed by the Georgia Tech Research Corp. based on the results in this study.
Figures





References
-
- Berríos-Torres SI, et al. Healthcare Infection Control Practices Advisory Committee Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–791. - PubMed
-
- Antoci V, Chen AF, Parvizi J. 7.9 Orthopedic implant use and infection. In: Ducheyne P, editor. Comprehensive Biomaterials II. Elsevier; Oxford: 2017. pp. 133–151.
-
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

