Characterization of the Anti-Hepatitis C Virus Activity of New Nonpeptidic Small-Molecule Cyclophilin Inhibitors with the Potential for Broad Anti-Flaviviridae Activity
- PMID: 29760125
- PMCID: PMC6021681
- DOI: 10.1128/AAC.00126-18
Characterization of the Anti-Hepatitis C Virus Activity of New Nonpeptidic Small-Molecule Cyclophilin Inhibitors with the Potential for Broad Anti-Flaviviridae Activity
Abstract
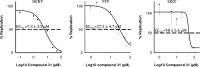
Although members of the Flaviviridae display high incidence, morbidity, and mortality rates, the development of specific antiviral drugs for each virus is unlikely. Cyclophilins, a family of host peptidyl-prolyl cis-trans isomerases (PPIases), play a pivotal role in the life cycles of many viruses and therefore represent an attractive target for broad-spectrum antiviral development. We report here the pangenotypic anti-hepatitis C virus (HCV) activity of a small-molecule cyclophilin inhibitor (SMCypI). Mechanistic and modeling studies revealed that the SMCypI bound to cyclophilin A in competition with cyclosporine (CsA), inhibited its PPIase activity, and disrupted the CypA-nonstructural protein 5A (NS5A) interaction. Resistance selection showed that the lead SMCypI hardly selected amino acid substitutions conferring low-level or no resistance in vitro Interestingly, the SMCypI selected D320E and Y321H substitutions, located in domain II of the NS5A protein. These substitutions were previously associated with low-level resistance to cyclophilin inhibitors such as alisporivir. Finally, the SMCypI inhibited the replication of other members of the Flaviviridae family with higher 50% effective concentrations (EC50s) than for HCV. Thus, because of its chemical plasticity and simplicity of synthesis, our new family of SMCypIs represents a promising new class of drugs with the potential for broad-spectrum anti-Flaviviridae activity as well as an invaluable tool to explore the role of cyclophilins in viral life cycles.
Keywords: Flaviviridae; antiviral agents; broad-spectrum antiviral activity; cyclophilin inhibitors; hepatitis C virus; resistance; small molecule.
Copyright © 2018 Nevers et al.
Figures
References
-
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. 2016. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 388:1081–1088. doi:10.1016/S0140-6736(16)30579-7. - DOI - PMC - PubMed
-
- World Health Organization. 2017. Global hepatitis report, 2017. World Health Organization, Geneva, Switzerland.
-
- Flisiak R, Horban A, Gallay P, Bobardt M, Selvarajah S, Wiercinska-Drapalo A, Siwak E, Cielniak I, Higersberger J, Kierkus J, Aeschlimann C, Grosgurin P, Nicolas-Metral V, Dumont JM, Porchet H, Crabbe R, Scalfaro P. 2008. The cyclophilin inhibitor Debio-025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology 47:817–826. doi:10.1002/hep.22131. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

