Developmental Sensitivity in Schistosoma mansoni to Puromycin To Establish Drug Selection of Transgenic Schistosomes
- PMID: 29760143
- PMCID: PMC6105839
- DOI: 10.1128/AAC.02568-17
Developmental Sensitivity in Schistosoma mansoni to Puromycin To Establish Drug Selection of Transgenic Schistosomes
Abstract
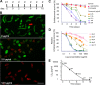
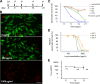
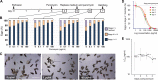
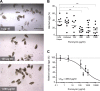
Schistosomiasis is considered the most important disease caused by helminth parasites, in terms of morbidity and mortality. Tools to facilitate gain- and loss-of-function approaches can be expected to precipitate the discovery of novel interventions, and drug selection of transgenic schistosomes would facilitate the establishment of stable lines of engineered parasites. Sensitivity of developmental stages of schistosomes to the aminonucleoside antibiotic puromycin was investigated. For the schistosomulum and sporocyst stages, viability was quantified by fluorescence microscopy following dual staining with fluorescein diacetate and propidium iodine. By 6 days in culture, the 50% lethal concentration (LC50) for schistosomula was 19 μg/ml whereas the sporocysts were 45-fold more resilient. Puromycin potently inhibited the development of in vitro-laid eggs (LC50, 68 ng/ml) but was less effective against liver eggs (LC50, 387 μg/ml). Toxicity for adult stages was evaluated using the xCELLigence-based, real-time motility assay (xWORM), which revealed LC50s after 48 h of 4.9 and 17.3 μg/ml for male and female schistosomes, respectively. Also, schistosomula transduced with pseudotyped retrovirus encoding the puromycin resistance marker were partially rescued when cultured in the presence of the antibiotic. Together, these findings will facilitate selection on puromycin of transgenic schistosomes and the enrichment of cultures of transgenic eggs and sporocysts to facilitate the establishment of schistosome transgenic lines. Streamlining schistosome transgenesis with drug selection will open new avenues to understand parasite biology and hopefully lead to new interventions for this neglected tropical disease.
Keywords: antibiotic susceptibility; drug selection; functional genomics; puromycin; schistosomiasis.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus.FASEB J. 2008 Aug;22(8):2936-48. doi: 10.1096/fj.08-108308. Epub 2008 Apr 10. FASEB J. 2008. PMID: 18403630 Free PMC article.
-
Germline transgenesis and insertional mutagenesis in Schistosoma mansoni mediated by murine leukemia virus.PLoS Pathog. 2012;8(7):e1002820. doi: 10.1371/journal.ppat.1002820. Epub 2012 Jul 26. PLoS Pathog. 2012. PMID: 22911241 Free PMC article.
-
An antibiotic selection marker for schistosome transgenesis.Int J Parasitol. 2012 Jan;42(1):123-30. doi: 10.1016/j.ijpara.2011.11.005. Epub 2011 Nov 29. Int J Parasitol. 2012. PMID: 22155152 Free PMC article.
-
Progress with schistosome transgenesis.Mem Inst Oswaldo Cruz. 2011 Nov;106(7):785-93. doi: 10.1590/s0074-02762011000700002. Mem Inst Oswaldo Cruz. 2011. PMID: 22124549 Free PMC article. Review.
-
Culture for genetic manipulation of developmental stages of Schistosoma mansoni.Parasitology. 2010 Mar;137(3):451-62. doi: 10.1017/S0031182009991211. Epub 2009 Sep 21. Parasitology. 2010. PMID: 19765348 Free PMC article. Review.
Cited by
-
The Transcriptome of Schistosoma mansoni Developing Eggs Reveals Key Mediators in Pathogenesis and Life Cycle Propagation.Front Trop Dis. 2021 Aug 18;2:713123. doi: 10.3389/fitd.2021.713123. Front Trop Dis. 2021. PMID: 36389622 Free PMC article.
-
Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni.Elife. 2019 Jan 15;8:e41337. doi: 10.7554/eLife.41337. Elife. 2019. PMID: 30644357 Free PMC article.
-
Biomphalaria glabrata immunity: Post-genome advances.Dev Comp Immunol. 2020 Mar;104:103557. doi: 10.1016/j.dci.2019.103557. Epub 2019 Nov 21. Dev Comp Immunol. 2020. PMID: 31759924 Free PMC article. Review.
-
Determining the viability of Schistosoma mansoni cercariae using fluorescence assays: An application for water treatment.PLoS Negl Trop Dis. 2020 Mar 26;14(3):e0008176. doi: 10.1371/journal.pntd.0008176. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32214320 Free PMC article.
-
Schistosomiasis Drug Discovery in the Era of Automation and Artificial Intelligence.Front Immunol. 2021 May 31;12:642383. doi: 10.3389/fimmu.2021.642383. eCollection 2021. Front Immunol. 2021. PMID: 34135888 Free PMC article. Review.
References
-
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. 2009. The genome of the blood fluke Schistosoma mansoni. Nature 460:352–358. doi:10.1038/nature08160. - DOI - PMC - PubMed
-
- Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, De Silva N, Velarde GS, Anderson TJ, Clark RC, Davidson C, Dillon GP, Holroyd NE, LoVerde PT, Lloyd C, McQuillan J, Oliveira G, Otto TD, Parker-Manuel SJ, Quail MA, Wilson RA, Zerlotini A, Dunne DW, Berriman M. 2012. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis 6:e1455. doi:10.1371/journal.pntd.0001455. - DOI - PMC - PubMed
-
- Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, Li Y, Cantacessi C, Hall RS, Xu X, Chen F, Wu X, Zerlotini A, Oliveira G, Hofmann A, Zhang G, Fang X, Kang Y, Campbell BE, Loukas A, Ranganathan S, Rollinson D, Rinaldi G, Brindley PJ, Yang H, Wang J, Gasser RB. 2012. Whole-genome sequence of Schistosoma haematobium. Nat Genet 44:221–225. doi:10.1038/ng.1065. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

