The role of IL-1B in breast cancer bone metastasis
- PMID: 29760166
- PMCID: PMC5987176
- DOI: 10.1530/ERC-17-0309
The role of IL-1B in breast cancer bone metastasis
Abstract
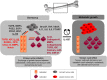
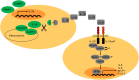
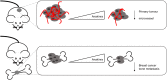
Approximately 75% of patients with late-stage breast cancer will develop bone metastasis. This condition is currently considered incurable and patients' life expectancy is limited to 2-3 years following diagnosis of bone involvement. Interleukin (IL)-1B is a pro-inflammatory cytokine whose expression in primary tumours has been identified as a potential biomarker for predicting breast cancer patients at increased risk for developing bone metastasis. In this review, we discuss how IL-1B from both the tumour cells and the tumour microenvironment influence growth of primary breast tumours, dissemination into the bone metastatic niche and proliferation into overt metastases. Recent evidence indicates that targeting IL-1B signalling may provide promising new treatments that can hold tumour cells in a dormant state within bone thus preventing formation of overt bone metastases.
Keywords: IL-1B; bone metastasis; breast cancer.
© 2018 The authors.
Figures
References
-
- Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, et al 2014. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nature Immunology 15 738–748. ( 10.1038/ni.2919) - DOI - PubMed
-
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. 2007. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Research 67 10019–10026. ( 10.1158/0008-5472.CAN-07-2354) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

