The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions
- PMID: 29760180
- PMCID: PMC6705939
- DOI: 10.1523/JNEUROSCI.0019-18.2018
The Affective and Neural Correlates of Heroin versus Cocaine Use in Addiction Are Influenced by Environmental Setting But in Opposite Directions
Abstract
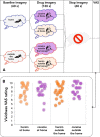
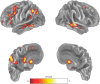
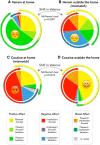
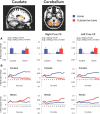
Previous studies have shown that individuals with heroin and cocaine addiction prefer to use these drugs in distinct settings: mostly at home in the case of heroin and mostly outside the home in the case of cocaine. Here we investigated whether the context would modulate the affective and neural responses to these drugs in a similar way. First, we used a novel emotional task to assess the affective state produced by heroin or cocaine in different settings, based on the recollections of male and female drug users. Then we used fMRI to monitor neural activity during drug imagery (re-creating the setting of drug use) in male drug users. Consistent with our working hypothesis, the majority of participants reported a shift in the affective valence of heroin from mostly pleasant at home to mostly unpleasant outside the home (p < 0.0001). The opposite shift was observed for cocaine; that is, most participants who found cocaine pleasant outside the home found it unpleasant when taken at home (p < 0.0014). Furthermore, we found a double dissociation, as a function of drug and setting imagery, in BOLD signal changes in the left PFC and caudate, and bilaterally in the cerebellum (all p values <0.01), suggesting that the fronto-striatal-cerebellar network is implicated in the contextualization of drug-induced affect. In summary, we report that the same setting can influence in opposite directions the affective and neural response to psychostimulants versus opiates in humans, adding to growing evidence of distinct substrates for the rewarding effects of these two drug classes.SIGNIFICANCE STATEMENT The rewarding effects of addictive drugs are often thought to depend on shared substrates. Yet, environmental influences can unmask striking differences between psychostimulants and opiates. Here we used emotional tasks and fMRI to explore the influence of setting on the response to heroin versus cocaine in individuals with addiction. Simply moving from one setting to another significantly decreased heroin pleasure but increased cocaine pleasure, and vice versa. Similar double dissociation was observed in the activity of the fronto-striatal-cerebellar network. These findings suggest that the effects of opiates and psychostimulants depend on dissociable psychological and neural substrates and that therapeutic approaches to addiction should take into account the peculiarities of different drug classes and the settings of drug use.
Keywords: addiction; context; emotion; fMRI; opiates; psychostimulants.
Copyright © 2018 the authors 0270-6474/18/385182-14$15.00/0.
Figures

Similar articles
-
Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study.Addict Biol. 2010 Oct;15(4):504-16. doi: 10.1111/j.1369-1600.2010.00230.x. Addict Biol. 2010. PMID: 20579005
-
Heroin versus cocaine: opposite choice as a function of context but not of drug history in the rat.Psychopharmacology (Berl). 2019 Feb;236(2):787-798. doi: 10.1007/s00213-018-5115-1. Epub 2018 Nov 15. Psychopharmacology (Berl). 2019. PMID: 30443795 Free PMC article.
-
Common and distinct fronto-striatal volumetric changes in heroin and cocaine use disorders.Brain. 2023 Apr 19;146(4):1662-1671. doi: 10.1093/brain/awac366. Brain. 2023. PMID: 36200376 Free PMC article.
-
Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex.Trends Pharmacol Sci. 2013 Dec;34(12):689-95. doi: 10.1016/j.tips.2013.10.004. Epub 2013 Oct 29. Trends Pharmacol Sci. 2013. PMID: 24182624 Review.
-
Role of environmental factors in cocaine addiction.Curr Pharm Des. 2013;19(40):6996-7008. doi: 10.2174/1381612819999131125221238. Curr Pharm Des. 2013. PMID: 23574438 Review.
Cited by
-
The Modulation of Reward and Habit Systems by Acupuncture in Adolescents with Internet Addiction.Neural Plast. 2020 Mar 13;2020:7409417. doi: 10.1155/2020/7409417. eCollection 2020. Neural Plast. 2020. PMID: 32256558 Free PMC article.
-
The role of social isolation in opioid addiction.Soc Cogn Affect Neurosci. 2021 Jul 6;16(7):645-656. doi: 10.1093/scan/nsab029. Soc Cogn Affect Neurosci. 2021. PMID: 33681992 Free PMC article.
-
Current understanding of the neurobiology of opioid use disorder: An overview.Curr Behav Neurosci Rep. 2019 Mar 15;6(1):1-11. doi: 10.1007/s40473-019-0170-4. Epub 2019 Jan 17. Curr Behav Neurosci Rep. 2019. PMID: 34485022 Free PMC article.
-
Rumination and drug craving scores in Chinese male patients with methamphetamine and heroin use disorders: a cross-sectional study.Subst Abuse Treat Prev Policy. 2025 Mar 26;20(1):14. doi: 10.1186/s13011-025-00643-z. Subst Abuse Treat Prev Policy. 2025. PMID: 40140962 Free PMC article.
-
The protective effect of operant social reward on cocaine self-administration, choice, and relapse is dependent on delay and effort for the social reward.Neuropsychopharmacology. 2021 Dec;46(13):2350-2357. doi: 10.1038/s41386-021-01148-6. Epub 2021 Aug 16. Neuropsychopharmacology. 2021. PMID: 34400784 Free PMC article.
References
-
- Adamaszek M, D'Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariën P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J (2017) Consensus paper: cerebellum and emotion. Cerebellum 16:552–576. 10.1007/s12311-016-0815-8 - DOI - PubMed
-
- Avvisati R, Contu L, Stendardo E, Michetti C, Montanari C, Scattoni ML, Badiani A (2016) Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: evidence of substance-specific interactions between drug and setting. Psychopharmacology 233:1501–1511. 10.1007/s00213-016-4247-4 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
