Structural and Functional Rescue of Chronic Metabolically Stressed Optic Nerves through Respiration
- PMID: 29760184
- PMCID: PMC5977447
- DOI: 10.1523/JNEUROSCI.3652-17.2018
Structural and Functional Rescue of Chronic Metabolically Stressed Optic Nerves through Respiration
Abstract
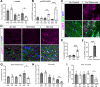
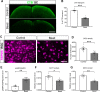
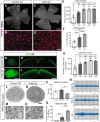
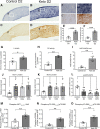
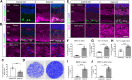
Axon degeneration can arise from metabolic stress, potentially a result of mitochondrial dysfunction or lack of appropriate substrate input. In this study, we investigated whether the metabolic vulnerability observed during optic neuropathy in the DBA/2J (D2) model of glaucoma is due to dysfunctional mitochondria or impaired substrate delivery to axons, the latter based on our observation of significantly decreased glucose and monocarboxylate transporters in D2 optic nerve (ON), human ON, and mice subjected to acute glaucoma injury. We placed both sexes of D2 mice destined to develop glaucoma and mice of a control strain, the DBA/2J-Gpnmb+, on a ketogenic diet to encourage mitochondrial function. Eight weeks of the diet generated mitochondria, improved energy availability by reversing monocarboxylate transporter decline, reduced glial hypertrophy, protected retinal ganglion cells and their axons from degeneration, and maintained physiological signaling to the brain. A robust antioxidant response also accompanied the response to the diet. These results suggest that energy compromise and subsequent axon degeneration in the D2 is due to low substrate availability secondary to transporter downregulation.SIGNIFICANCE STATEMENT We show axons in glaucomatous optic nerve are energy depleted and exhibit chronic metabolic stress. Underlying the metabolic stress are low levels of glucose and monocarboxylate transporters that compromise axon metabolism by limiting substrate availability. Axonal metabolic decline was reversed by upregulating monocarboxylate transporters as a result of placing the animals on a ketogenic diet. Optic nerve mitochondria responded capably to the oxidative phosphorylation necessitated by the diet and showed increased number. These findings indicate that the source of metabolic challenge can occur upstream of mitochondrial dysfunction. Importantly, the intervention was successful despite the animals being on the cusp of significant glaucoma progression.
Keywords: b-hydroxybutyrate; ketogenic diet; neural-glial interaction; optic nerve.
Copyright © 2018 the authors 0270-6474/18/385122-18$15.00/0.
Figures



Similar articles
-
Higher Reliance on Glycolysis Limits Glycolytic Responsiveness in Degenerating Glaucomatous Optic Nerve.Mol Neurobiol. 2019 Oct;56(10):7097-7112. doi: 10.1007/s12035-019-1576-4. Epub 2019 Apr 13. Mol Neurobiol. 2019. PMID: 30980229 Free PMC article.
-
Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma.J Neuroinflammation. 2018 Nov 13;15(1):313. doi: 10.1186/s12974-018-1346-7. J Neuroinflammation. 2018. PMID: 30424795 Free PMC article.
-
Glial coverage in the optic nerve expands in proportion to optic axon loss in chronic mouse glaucoma.Exp Eye Res. 2016 Sep;150:34-43. doi: 10.1016/j.exer.2016.01.014. Epub 2016 Feb 3. Exp Eye Res. 2016. PMID: 26851485 Free PMC article.
-
Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss.Exp Eye Res. 2015 Dec;141:111-24. doi: 10.1016/j.exer.2015.06.006. Epub 2015 Jun 9. Exp Eye Res. 2015. PMID: 26070986 Review.
-
Autophagy in axonal degeneration in glaucomatous optic neuropathy.Prog Retin Eye Res. 2015 Jul;47:1-18. doi: 10.1016/j.preteyeres.2015.03.002. Epub 2015 Mar 26. Prog Retin Eye Res. 2015. PMID: 25816798 Review.
Cited by
-
Transcorneal Electrical Stimulation Reduces Neurodegenerative Process in a Mouse Model of Glaucoma.Ann Biomed Eng. 2021 Feb;49(2):858-870. doi: 10.1007/s10439-020-02608-8. Epub 2020 Sep 24. Ann Biomed Eng. 2021. PMID: 32974756 Free PMC article.
-
A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice.Aging (Albany NY). 2021 Mar 18;13(6):7914-7930. doi: 10.18632/aging.202834. Epub 2021 Mar 18. Aging (Albany NY). 2021. PMID: 33735837 Free PMC article.
-
The Role of Axonal Transport in Glaucoma.Int J Mol Sci. 2022 Apr 1;23(7):3935. doi: 10.3390/ijms23073935. Int J Mol Sci. 2022. PMID: 35409291 Free PMC article. Review.
-
Aging Effects on Optic Nerve Neurodegeneration.Int J Mol Sci. 2023 Jan 29;24(3):2573. doi: 10.3390/ijms24032573. Int J Mol Sci. 2023. PMID: 36768896 Free PMC article. Review.
-
Neuroprotection by the Ketogenic Diet: Evidence and Controversies.Front Nutr. 2021 Nov 23;8:782657. doi: 10.3389/fnut.2021.782657. eCollection 2021. Front Nutr. 2021. PMID: 34888340 Free PMC article. Review.
References
-
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 100:6216–6220. 10.1073/pnas.1035720100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
