Effects of Influenza on Alveolar Macrophage Viability Are Dependent on Mouse Genetic Strain
- PMID: 29760191
- PMCID: PMC6008236
- DOI: 10.4049/jimmunol.1701406
Effects of Influenza on Alveolar Macrophage Viability Are Dependent on Mouse Genetic Strain
Abstract
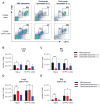
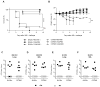
Secondary bacterial coinfections following influenza virus pose a serious threat to human health. Therefore, it is of significant clinical relevance to understand the immunological causes of this increased susceptibility. Influenza-induced alterations in alveolar macrophages (AMs) have been shown to be a major underlying cause of the increased susceptibility to bacterial superinfection. However, the mechanisms responsible for this remain under debate, specifically in terms of whether AMs are depleted in response to influenza infection or are maintained postinfection, but with disrupted phagocytic activity. The data presented in this article resolves this issue by showing that either mechanism can differentially occur in individual mouse strains. BALB/c mice exhibited a dramatic IFN-γ-dependent reduction in levels of AMs following infection with influenza A, whereas AM levels in C57BL/6 mice were maintained throughout the course of influenza infection, although the cells displayed an altered phenotype, namely an upregulation in CD11b expression. These strain differences were observed regardless of whether infection was performed with low or high doses of influenza virus. Furthermore, infection with either the H1N1 A/California/04/2009 (CA04) or H1N1 A/PR8/1934 (PR8) virus strain yielded similar results. Regardless of AM viability, both BALB/c and C57BL/6 mice showed a high level of susceptibility to postinfluenza bacterial infection. These findings resolve the apparent inconsistencies in the literature, identify mouse strain-dependent differences in the AM response to influenza infection, and ultimately may facilitate translation of the mouse model to clinical application.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors declare no competing financial interests
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

