Specific Binding to Differentially Expressed Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules Determines the Outcome of Neisseria gonorrhoeae Infections along the Female Reproductive Tract
- PMID: 29760215
- PMCID: PMC6056862
- DOI: 10.1128/IAI.00092-18
Specific Binding to Differentially Expressed Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules Determines the Outcome of Neisseria gonorrhoeae Infections along the Female Reproductive Tract
Abstract
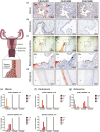
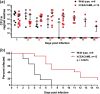
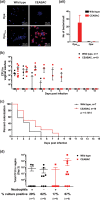
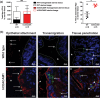
The gonococcal Opa proteins are an antigenically variable family of surface adhesins that bind human carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), CEACAM3, CEACAM5, and/or CEACAM6, cell surface glycoproteins that are differentially expressed on a broad spectrum of human cells and tissues. While they are presumed to be important for infection, the significance of various Opa-CEACAM-mediated cellular interactions in the context of the genital tract has remained unclear. Here, we observed that CEACAM1 and CEACAM5 are differentially expressed on epithelia lining the upper and lower portions of the human female genital tract, respectively. Using transgenic mouse lines expressing human CEACAMs in a manner that reflects this differential pattern, we considered the impact of Opa-CEACAM interactions during uncomplicated lower genital tract infections versus during pelvic inflammatory disease. Our results demonstrate that Opa-CEACAM5 binding on vaginal epithelia facilitates the long-term colonization of the lower genital tract, while Opa protein binding to CEACAM1 on uterine epithelia enhances gonococcal association and penetration into these tissues. While these Opa-dependent interactions with CEACAM-expressing epithelial surfaces promote infection, Opa binding by neutrophil-expressed CEACAMs counterbalances this by facilitating more effective gonococcal clearance. Furthermore, during uterine infections, CEACAM-dependent tissue invasion aggravates disease pathology by increasing the acute inflammatory response. Together, these findings demonstrate that the outcome of infection is determined by both the cell type-specific expression of human CEACAMs and the CEACAM specificity of the Opa variants expressed, which combine to determine the level of gonococcal association with the genital mucosa versus the extent of CEACAM-dependent inflammation and gonococcal clearance by neutrophils.
Keywords: CEACAM1; Neisseria gonorrhoeae; carcinoembryonic antigen (CEA); carcinoembryonic antigen-related cellular adhesion molecules (CEACAM); gonorrhea; inflammation; mucosal infection; pelvic inflammatory disease (PID); sexually transmitted disease (STD).
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract.Infect Immun. 2015 Apr;83(4):1372-83. doi: 10.1128/IAI.03123-14. Epub 2015 Jan 20. Infect Immun. 2015. PMID: 25605771 Free PMC article.
-
Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae.Infect Immun. 2012 Jan;80(1):345-58. doi: 10.1128/IAI.05702-11. Epub 2011 Nov 7. Infect Immun. 2012. PMID: 22064717 Free PMC article.
-
Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections.Infect Immun. 2013 Jul;81(7):2358-70. doi: 10.1128/IAI.00128-13. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630956 Free PMC article.
-
Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria.FEMS Microbiol Rev. 2011 May;35(3):498-514. doi: 10.1111/j.1574-6976.2010.00260.x. Epub 2011 Jan 17. FEMS Microbiol Rev. 2011. PMID: 21204865 Review.
-
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis.Cancer Metastasis Rev. 2013 Dec;32(3-4):643-71. doi: 10.1007/s10555-013-9444-6. Cancer Metastasis Rev. 2013. PMID: 23903773 Review.
Cited by
-
CEACAM3-A Prim(at)e Invention for Opsonin-Independent Phagocytosis of Bacteria.Front Immunol. 2020 Feb 11;10:3160. doi: 10.3389/fimmu.2019.03160. eCollection 2019. Front Immunol. 2020. PMID: 32117212 Free PMC article. Review.
-
Neisseria gonorrhoeae vaccines: a contemporary overview.Clin Microbiol Rev. 2024 Mar 14;37(1):e0009423. doi: 10.1128/cmr.00094-23. Epub 2024 Jan 16. Clin Microbiol Rev. 2024. PMID: 38226640 Free PMC article. Review.
-
Meningococcal vaccine 4CMenB elicits a robust cellular immune response that targets but is not consistently protective against Neisseria gonorrhoeae during murine vaginal infection.mSphere. 2025 May 27;10(5):e0094024. doi: 10.1128/msphere.00940-24. Epub 2025 Apr 16. mSphere. 2025. PMID: 40237483 Free PMC article.
-
CEACAM1 in Liver Injury, Metabolic and Immune Regulation.Int J Mol Sci. 2018 Oct 11;19(10):3110. doi: 10.3390/ijms19103110. Int J Mol Sci. 2018. PMID: 30314283 Free PMC article. Review.
-
Gonococcal Pelvic Inflammatory Disease: Placing Mechanistic Insights Into the Context of Clinical and Epidemiological Observations.J Infect Dis. 2021 Aug 16;224(12 Suppl 2):S56-S63. doi: 10.1093/infdis/jiab227. J Infect Dis. 2021. PMID: 34396410 Free PMC article. Review.
References
-
- Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O'Connell C, Boden R, Elkins C, Pangburn MK, Dahlback B, Rice PA. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med 193:281–295. doi:10.1084/jem.193.3.281. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

