Two distinct mechanisms for experience-dependent homeostasis
- PMID: 29760525
- PMCID: PMC6019646
- DOI: 10.1038/s41593-018-0150-0
Two distinct mechanisms for experience-dependent homeostasis
Abstract
Models of firing rate homeostasis such as synaptic scaling and the sliding synaptic plasticity modification threshold predict that decreasing neuronal activity (for example, by sensory deprivation) will enhance synaptic function. Manipulations of cortical activity during two forms of visual deprivation, dark exposure (DE) and binocular lid suture, revealed that, contrary to expectations, spontaneous firing in conjunction with loss of visual input is necessary to lower the threshold for Hebbian plasticity and increase miniature excitatory postsynaptic current (mEPSC) amplitude. Blocking activation of GluN2B receptors, which are upregulated by DE, also prevented the increase in mEPSC amplitude, suggesting that DE potentiates mEPSCs primarily through a Hebbian mechanism, not through synaptic scaling. Nevertheless, NMDA-receptor-independent changes in mEPSC amplitude consistent with synaptic scaling could be induced by extreme reductions of activity. Therefore, two distinct mechanisms operate within different ranges of neuronal activity to homeostatically regulate synaptic strength.
Conflict of interest statement
Figures

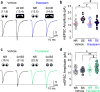
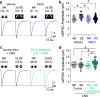



Comment in
-
Synaptic homeostasis: quality vs. quantity.Nat Neurosci. 2018 Jun;21(6):774-776. doi: 10.1038/s41593-018-0159-4. Nat Neurosci. 2018. PMID: 29760526 Free PMC article.
References
-
- Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–46. - PubMed
-
- Davis GW. Homeostatic control of neural activity: From phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. - PubMed
-
- Cooper LN, Bear MF. The BCM theory of synapse modification at 30: Interaction of theory with experiment. Nat Rev Neurosci. 2012;13:798–810. - PubMed
-
- Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

