Platelets in Immune Response to Virus and Immunopathology of Viral Infections
- PMID: 29761104
- PMCID: PMC5936789
- DOI: 10.3389/fmed.2018.00121
Platelets in Immune Response to Virus and Immunopathology of Viral Infections
Abstract
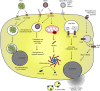
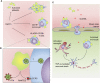
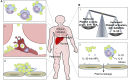
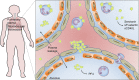
Platelets are essential effector cells in hemostasis. Aside from their role in coagulation, platelets are now recognized as major inflammatory cells with key roles in the innate and adaptive arms of the immune system. Activated platelets have key thromboinflammatory functions linking coagulation to immune responses in various infections, including in response to virus. Recent studies have revealed that platelets exhibit several pattern recognition receptors (PRR) including those from the toll-like receptor, NOD-like receptor, and C-type lectin receptor family and are first-line sentinels in detecting and responding to pathogens in the vasculature. Here, we review the main mechanisms of platelets interaction with viruses, including their ability to sustain viral infection and replication, their expression of specialized PRR, and activation of thromboinflammatory responses against viruses. Finally, we discuss the role of platelet-derived mediators and platelet interaction with vascular and immune cells in protective and pathophysiologic responses to dengue, influenza, and human immunodeficiency virus 1 infections.
Keywords: HIV infection; dengue; immunology and virology; influenza; platelet; viruses.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

