A trial like ALIC4E: why design a platform, response-adaptive, open, randomised controlled trial of antivirals for influenza-like illness?
- PMID: 29761108
- PMCID: PMC5938489
- DOI: 10.1183/23120541.00046-2018
A trial like ALIC4E: why design a platform, response-adaptive, open, randomised controlled trial of antivirals for influenza-like illness?
Abstract
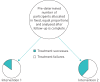
ALIC4E is the first publicly funded, multicountry, pragmatic study determining whether antivirals should be routinely prescribed for influenza-like illness in primary care. The trial aims to go beyond determining the average treatment effect in a population to determining effects in patients with combinations of participant characteristics (age, symptom duration, illness severity, and comorbidities). It is one of the first platform, response-adaptive, open trial designs implemented in primary care, and this article aims to provide an accessible description of key aspects of the study design. 1) The platform design allows the study to remain relevant to evolving circumstances, with the ability to add treatment arms. 2) Response adaptation allows the proportion of participants with key characteristics allocated to study arms to be altered during the course of the trial according to emerging outcome data, so that participants' information will be most useful, and increasing their chances of receiving the trial intervention that will be most effective for them. 3) Because the possibility of taking placebos influences participant expectations about their treatment, and determining effects of the interventions on patient help seeking and adherence behaviour in real-world care is critical to estimates of cost-effectiveness, ALIC4E is an open-label trial.
Conflict of interest statement
Conflict of interest: M. de Jong reports advisory board, travel and fees from Janssen, MedImmune and Shionogi. He also reports Independent Data and Safety Monitoring Board (IDSMB) and fees from Janssen, and IDSMB, travel and fees from GSK and Vertex, outside the submitted work. Conflict of interest: P. Beutels reports grants from European Commission project “PREPARE” during the conduct of the study, and an unrestricted gift for part-time research from Pfizer and GSK, outside the submitted work.
Figures

References
-
- Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385: 1729–1737. - PubMed
-
- Krumholz HM. Neuraminidase inhibitors for influenza. BMJ 2014; 348: g2548. - PubMed
-
- Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 2015; 313: 1619–1620. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
