Distinct timing of neurogenesis of ipsilateral and contralateral retinal ganglion cells
- PMID: 29761490
- PMCID: PMC6237670
- DOI: 10.1002/cne.24467
Distinct timing of neurogenesis of ipsilateral and contralateral retinal ganglion cells
Abstract
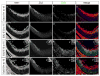
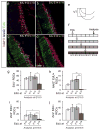
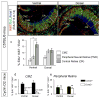
In higher vertebrates, the circuit formed by retinal ganglion cells (RGCs) projecting ipsilaterally (iRGCs) or contralaterally (cRGCs) to the brain permits binocular vision and depth perception. iRGCs and cRGCs differ in their position within the retina and in expression of transcription, guidance and activity-related factors. To parse whether these two populations also differ in the timing of their genesis, a feature of distinct neural subtypes and associated projections, we used newer birthdating methods and cell subtype specific markers to determine birthdate and cell cycle exit more precisely than previously. In the ventrotemporal (VT) retina, i- and cRGCs intermingle and neurogenesis in this zone lags behind RGC production in the rest of the retina where only cRGCs are positioned. In addition, within the VT retina, i- and cRGC populations are born at distinct times: neurogenesis of iRGCs surges at E13, and cRGCs arise as early as E14, not later in embryogenesis as reported. Moreover, in the ventral ciliary margin zone (CMZ), which contains progenitors that give rise to some iRGCs in ventral neural retina (Marcucci et al., 2016), cell cycle exit is slower than in other retinal regions in which progenitors give rise only to cRGCs. Further, when the cell cycle regulator Cyclin D2 is missing, cell cycle length in the CMZ is further reduced, mirroring the reduction of both i- and cRGCs in the Cyclin D2 mutant. These results strengthen the view that differential regulation of cell cycle dynamics at the progenitor level is associated with specific RGC fates and laterality of axonal projection.
Keywords: RRID: AB_2315623; RRID: AB_2534079; RRID: AB_2535812; RRID: AB_2556549; RRID: AB_443209; RRID: AB_528173; binocular vision; contralateral RGCs; ipsilateral RGCs; neurogenesis; retina.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest: The authors declare no competing financial interests.
Figures
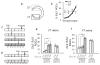
Similar articles
-
Delayed neurogenesis leads to altered specification of ventrotemporal retinal ganglion cells in albino mice.Neural Dev. 2014 May 18;9:11. doi: 10.1186/1749-8104-9-11. Neural Dev. 2014. PMID: 24885435 Free PMC article.
-
The Ciliary Margin Zone of the Mammalian Retina Generates Retinal Ganglion Cells.Cell Rep. 2016 Dec 20;17(12):3153-3164. doi: 10.1016/j.celrep.2016.11.016. Cell Rep. 2016. PMID: 28009286 Free PMC article.
-
Shh/Boc signaling is required for sustained generation of ipsilateral projecting ganglion cells in the mouse retina.J Neurosci. 2013 May 15;33(20):8596-607. doi: 10.1523/JNEUROSCI.2083-12.2013. J Neurosci. 2013. PMID: 23678105 Free PMC article.
-
Wiring the Binocular Visual Pathways.Int J Mol Sci. 2019 Jul 4;20(13):3282. doi: 10.3390/ijms20133282. Int J Mol Sci. 2019. PMID: 31277365 Free PMC article. Review.
-
Neurogenesis and Specification of Retinal Ganglion Cells.Int J Mol Sci. 2020 Jan 10;21(2):451. doi: 10.3390/ijms21020451. Int J Mol Sci. 2020. PMID: 31936811 Free PMC article. Review.
Cited by
-
CyclinD2-mediated regulation of neurogenic output from the retinal ciliary margin is perturbed in albinism.Neuron. 2023 Jan 4;111(1):49-64.e5. doi: 10.1016/j.neuron.2022.10.025. Epub 2022 Nov 8. Neuron. 2023. PMID: 36351424 Free PMC article.
-
Reanalysis of EphA3 Knock-In Double Maps in Mouse Suggests That Stochasticity in Topographic Map Formation Acts at the Retina Rather than between Competing Mechanisms at the Colliculus.eNeuro. 2023 Nov 21;10(11):ENEURO.0135-23.2023. doi: 10.1523/ENEURO.0135-23.2023. Print 2023 Nov. eNeuro. 2023. PMID: 37852780 Free PMC article.
-
Decreased CREB phosphorylation impairs embryonic retinal neurogenesis in the Oa1-/- mouse model of Ocular albinism.bioRxiv [Preprint]. 2024 May 14:2024.05.14.594013. doi: 10.1101/2024.05.14.594013. bioRxiv. 2024. PMID: 38798688 Free PMC article. Preprint.
-
CXCL12 promotes the crossing of retinal ganglion cell axons at the optic chiasm.Development. 2024 Jan 15;151(2):dev202446. doi: 10.1242/dev.202446. Epub 2024 Jan 15. Development. 2024. PMID: 38095299 Free PMC article.
-
Retinal ganglion cell circuits and glial interactions in humans and mice.Trends Neurosci. 2024 Dec;47(12):994-1013. doi: 10.1016/j.tins.2024.09.010. Epub 2024 Oct 24. Trends Neurosci. 2024. PMID: 39455342 Review.
References
-
- Agathocleous M, Locker M, Harris WA, Perron M. A general role of hedgehog in the regulation of proliferation. Cell Cycle. 2007;6(2):156–159. 3745 [pii] - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

